
Quantification of Imidazole Compounds in Ambient Aerosols at Suburban and Forest Sites in Western Japan
Copyright © 2019 by Asian Journal of Atmospheric Environment
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Ambient aerosol particles at forest and suburban sites in western Japan were analyzed for imidazole compounds, such as 4 (5)-Methylimidazole (4-MI), 1-ethylimidazole (1-EI), 2-ethylimidazole (2-EI), and imidazole-2-carboxaldehyde (IC). The aerosols were collected on quartz fiber filters and extracted by the solid phase extraction method. The extract was analyzed by HPLC/Q-TOF-MS using an ion-pairing agent. The concentration of 4-MI in winter was higher than those in summer at the forest site; its concentration was highest among the analyzed imidazoles. The concentration of 4-MI in the suburban site was several times higher than the forest site. Anthropogenic activities, such as vehicle emissions, are considered to be the major source of 4-MI in this region.
Keywords:
Imidazole compounds, Methylimidazole, Ambient, Quantification, HPLC/ Q-TOF-MS, Ion-pairing agent, Aerosol particle1. INTRODUCTION
Imidazole-structured compounds are formed by the interaction of ammonia with reducing sugars (Moon and Shibamoto, 2011). Therefore, they are often seen as byproducts in some foods and beverages (Pflaum et al., 2016) during the normal cooking process of heating and browning. Foods and beverages, such as soy sauce, wine, dark beers, soft drinks, coffee, bread, and baked goods, contain these chemicals, which have caramel color (Vollmuth, 2018). 4 (5)-Methylimidazole (4-MI) is known for its carcinogenicity and is classified as group 2B carcinogen (“possibly carcinogenic to humans”) by the International Agency for Research on Cancer (IARC). Human health is one of the most important research concerns; therefore, there are many reports on the detection (Wu et al., 2019; Vollmuth, 2018; Pflaum et al., 2016; Yamaguchi and Masuda, 2011) or evaluation (Morita and Uneyama, 2016) of 4-MI in foods and beverages.
In recent years, scientific interest in brown carbon, and light absorbing organic aerosols and particles in the atmosphere has been increasing due to their possible contributions to radiation balance and cloud formation (Laskin et al., 2015; Feng et al., 2013). Brown carbon is a part of organic carbon that accounts for a large fraction of atmospheric aerosols, and has a significant light absorption at near UV and shorter visible wavelengths. The sources of brown carbon which were biomass burning, fossil fuel combustion and secondary formation in the atmosphere have been reported ( Jiang et al., 2019; Laskin et al., 2015; Saleh et al., 2013; Updyke et al., 2012; Nakayama et al., 2010; Lukács et al., 2007). Many types of chromophores, such as polycyclic aromatics, humic-like substances, nitro-aromatic compounds, and imidazole compounds and their sources have been proposed (Jiang et al., 2019; Laskin et al., 2015; Saleh et al., 2013; Updyke et al., 2012; Nakayama et al., 2010; Lukács et al., 2007). Many laboratory studies reported the formation of imidazoles during aqueous-phase or heterogeneous reactions involving glyoxal or methylglyoxal with ammonium or gaseous ammonia (Huang et al., 2018; Lin et al., 2015; Kampf et al., 2012; De Haan et al., 2009). However, to the best of our knowledge, there is only one study that reports the detection of imidazoles in ambient aerosol particles (Teich et al., 2016). Teich et al. (2016) quantified several imidazole compounds in ambient particles collected in Germany, Italy, and China.
The objectives of the present study were to (a) develop a protocol to analyze imidazole compounds, (b) determine imidazole concentration levels at suburban and forest sites in western Japan, and (c) understand the possible sources of imidazoles in the study regions.
2. EXPERIMENTAL
2. 1 Imidazole Standards
Four imidazole compounds, including 4 (5)-Methylimidazole (4-MI), 1-ethylimidazole (1-EI), 2-ethylimidazole (2-EI), and imidazole-2-carboxaldehyde (IC) were selected for quantification in this study by referencing to the report of Teich et al. (2016). Standard reagents of 4-MI, 1-EI, and IC were purchased from Oakwood Products, Inc. (SC., USA). The 2-EI was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The 4 (5)-Methylimidazole-d3 (4-MI-d3) was used as a surrogate compound for precise measurement, and it was purchased from Nacalai Tesque, Inc. (Kyoto, Japan). The standard solutions for these compounds were prepared by dilution with pure water.
2. 2 Aerosol Sampling and Pretreatment
Quartz fiber filters (254 mm×203 mm, QR-100, Advantec, Tokyo, Japan) were preheated at 800°C for 8 hours and weighed after drying in a desiccator for >24 hours. The quartz fiber filters (8ʺ×10ʺ) were used for collecting aerosols with a high-volume sampler (HV-7000F, Sibata Scientific Technology Ltd., Saitama, Japan). The total suspended aerosol particles were collected, without size selection, by operating the high-volume sampler at a flow rate of 700 L/min. Collection of the aerosol particles were carried out at a weekly interval in summer, from July 18 to August 25, 2018, and in winter from December 19, 2018 to January 7, 2019 at the forest site, and from January 9, 2019 to January 23, 2019 at the suburban site. The sampling time was one week. On average, one rainy day was experienced during the one week sampling periods in both seasons with averaged accumulated rainfalls of 13.6 and 5.5 mm during the one week sampling periods in summer and winter, respectively.
The forest site (32°54ʹ32.4ʺN, 129°44ʹ33.5ʺE, 533 m above sea level) was at Nagasaki prefectural forest park (24 km northeast from Nagasaki city center) near the top of the Miharashi mountain in Nishisonogi peninsula and surrounded mainly by deciduous trees. The peninsula has steep terrain, few flats, low population density, and it faces the East of the China Sea. The site is considered to be comparatively isolated from the influences of anthropogenic pollution sources. The suburban site (32°47ʹ07.9ʺN, 129°51ʹ52.6ʺE, 20 m above sea level) was located at Bunkyo Campus of Nagasaki University. The sampling was conducted on the roof of a four-storied building on the campus. The campus was surrounded by residential and business areas in Nagasaki city with a population of 0.4 million. The industrial area is located around Nagasaki Port, which is 5 km south from the suburban site.
After the filters were kept in a desiccator for several days, the filters were weighed (Table S1) and stored at -20°C until extraction. Aliquots of the aerosol particle samples in the filter were extracted with 100 mL ultrapure water containing 1 μg of 4-MI-d3 as a surrogate (internal recovery standard). After sonication for 20 min, the extract was filtered through a cartridge with a pore size of 0.45 μm. Oasis MCX plus short cartridge, 225 mg sorbent, (Waters Corp. MA, USA) was used for the solid phase extraction with reference to Teich et al. (2016). The cartridge had a reversed phase mode, and cation exchange sorbents were expected to sorb the imidazoles, which have high water solubility. The cartridge was prewashed with water-methanol mixture (1:1, v/v) and water before using for the solid phase extraction. The aqueous solution of the sample was adjusted to a pH of 2 with H2SO4 and passed through the cartridge. The cartridge was then washed with 5 mL of 2% formic acid solution, followed by 5 mL methanol to remove undesired impurities having low polarity. The imidazoles retained by the cation exchange sorbents were eluted from the cartridge using 1 mL of methanol containing 5% ammonia. The extracted solution was exsiccated by gently blowing nitrogen gas, and 1 mL water was added before the sample was analyzed with High-Performance Liquid Chromatography (HPLC)/Quadrupole Time-Of-Flight (TOF) mass spectrometer.
2. 3 Instrumentation
The analysis was carried out by an HPLC (1290 Infinity, Agilent Technologies, Inc., CA, USA) connected with Q-TOF-MS (6550 iFunnel, Agilent Technologies, Inc.). Chromatographic separation was performed using Eclipse Plus C18, 2.1×100 mm, 1.8 μm analytical column (Agilent Technologies, Inc.) under a gradient program. The initial mobile phase condition consisted of 5 mM heptafluorobutyric acid as an ion-pairing agent in water and acetonitrile (100:0, v/v). A linear gradient was applied at a ratio of 80:20 over 8 mins to analyze imidazoles. The ratio was then changed to 0:100 for cleaning, and it was maintained for 11 min. The flow rate was 0.3 mL/min, and the injection volume was 1 μL.
The electrospray ionization (ESI) conditions were as follows: ionization mode was ESI+, dry gas temperature was 200°C, dry gas flow rate was 14 L/min, sheath gas temperature was 350°C, sheath gas flow rate was 11 L/min, and nozzle voltage was 1000 V. The detailed measurement conditions for each compound are shown in Table 1. The protonated ions of target imidazoles were passed through the quadrupole type mass filter with low resolution, and they were decomposed partially in the collision cell. Their accurate mass was analyzed with the TOF-MS. For each imidazole compound, the retention time, limits of detection (LOD), and limits of quantification (LOQ), calculated with signal-to-noise (S/N) ratios of 3 and 10 for LOD and LOQ, respectively, are summarized in Table 1.
3. RESULTS AND DISCUSSION
The HPLC/Q-TOF-MS chromatogram of the standard solution of imidazoles mixture is shown in Figure 1. The injected mass weighed between 1.0 and 2.4 ng. Despite the high water solubility of imidazoles and the use of a C18 reverse phase column, their peaks could be seen sharply by using an ion-pairing reagent. Since, 1-EI and 2-EI had the same molecular weight as shown in Table 1, their peaks appeared at a close retention time. There were two peaks of 1-EI as shown in Fig. 1 (c), however the first one was 1-EI and the second one was 2-EI. Only the first peak was used when measuring the 1-EI area. These compounds had different fragmentation patterns despite their identical molecular weight and one of the merits of MS-MS analysis was visible here.
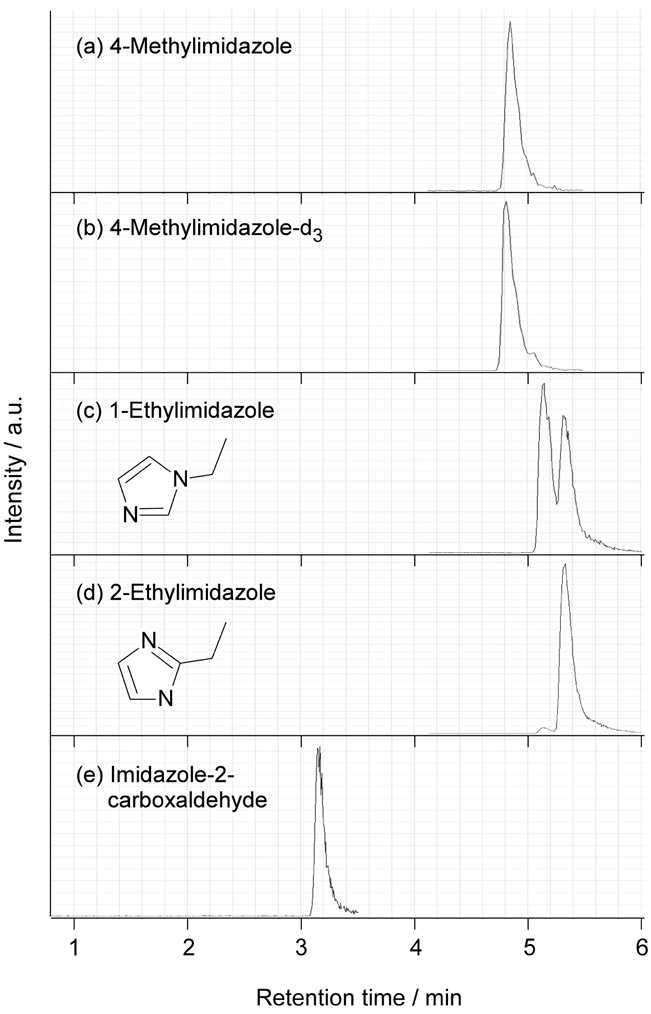
HPLC/Q-TOF-MS chromatogram of imidazoles mixture analyzed with 5 mM heptafluorobutyric acid solution as an ionpairing agent. The injected mass of each chemicals of (a) to (e) were 2.4, 2.0, 1.0, 1.0 and 1.4 ng, respectively.
The mass concentrations and mass fraction of imidazoles in aerosol particles collected at both sites are listed in Table 2. The total aerosol mass concentrations, to obtain mass fraction, were calculated from the weights of the filters (before and after sampling), and the total sampling volume calculated from the flow rate and sampling time. Although meteorological conditions, involving the stability of the atmosphere and boundary layer height, strongly influence mass concentrations of particulates, the effect of meteorological conditions on the mass fractions of imidazoles should be minimal.
Concentrations of imidazoles in aerosol collected at the forest and sub-urban sites in Nagasaki Prefecture, Japan in summer and winter.
The 4-MI, 1-EI, and 2-EI were detected in winter season at the sub-urban site. The 4-MI was successfully quantified in all aerosol samples. The IC peak was not found in all samples. This ordering of concentrations was quite consistent with the results of Teich et al. (2016), in which 4-MI showed the highest concentrations followed by EIs, and the detection frequency of IC was low. Our results suggest that 4-MI is one of the major imidazole compounds in ambient particles. Considering the high toxicity of 4-MI (Vollmuth, 2018; Morita and Uneyama, 2016; Pflaum et al., 2016), continuous monitoring of 4-MI is required. The mass concentrations of 4-MI in this study were one or two orders of magnitudes lower than those reported by Teich et al. (2016). They suggested that the main source of imidazoles at the TROPOS site, where high concentrations of 4-MI was observed, was biomass burning based on the correlation of 4-MI with K+, which is a tracer of biomass burning, and it was also known that imidazoles produced by biomass burning in laboratory studies (Dou et al., 2015; Laskin et al., 2009). The reason for the lower concentrations of 4-MI observed in this study may partly be attributed to the relatively low contribution of biomass burning, considering biofuels are rarely used for cooking and heating in the area.
The concentrations and fractions of 4-MI at the forest site were several times higher in the winter than those in the summer. Several factors could contribute to the observed seasonal differences: higher contributions of transboundary air mass from the Asian continent in winter (Kaneyasu et al., 2014), higher contributions of agricultural residue burning in winter (Tomiyama et al., 2017), and slower photochemical degradation of imidazoles in winter. The possible contribution of the variation of gas-to-particle partitioning cannot be ruled out, because their vapor pressures are expected to be rather low in winter. Furthermore, decomposition or formation of imidazoles on sampling filter during the sampling periods might also affect to the results. Teich et al. (2016) reported that no apparent difference in the concentrations of 4-MI between summer and winter was found at the Melpitz site in Germany. Further studies, including the simultaneous observation of imidazoles and other compositions in aerosol particles, as well as gaseous precursors, is required to understand the source of the observed seasonal variations in this study.
Measurement were made at both suburban and forest sites in the winter. As listed in Table 2, the concentrations and fractions of 4-MI at the suburban site were 3-5 times higher than those at the forest site. This result suggests that the major source (s) of imidazoles in the area could be related to human activities, such as emissions from vehicles (e.g., cars, buses, trucks, and motorcycles) and industries in the Nagasaki city. Teich et al. (2016) also reported a connection between high concentrations of imidazoles and highly polluted air mass from urbanized area at the Wuqing site in China.
Many laboratory studies (Huang et al., 2018; Hamilton et al., 2013; Kampf et al., 2012) reported the formation of imidazoles in secondary organic aerosols (SOA) through an aqueous phase or heterogeneous reactions involving ammonium ions or ammonia. Recently, it was indicated that vehicular exhaust contributed to high ammonia in the atmosphere in Tokyo (Osada et al., 2019). Secondary formation of imidazoles through reactions with ammonia emitted from vehicles, especially having three-way catalytic converters or selective catalytic reduction system, or direct emission of imidazoles from these vehicles might contribute to higher concentrations observed at the suburban site. This study demonstrates the importance of anthropogenic sources of imidazoles, especially in the urban area.
4. CONCLUSIONS
4-MI was quantified with the highest concentration in all aerosol samples. The second highest concentrations observed were for 1- and 2-ethylimidazoles. The values noted for the species were lower than concentrations previously reported for other countries because the region concern experiences relatively lower rates of biomass burning. The concentrations of 4-MI at the suburban site were 3-5 times higher than those in the forest site. The major source of imidazoles in suburban area was suggested to be related to human activities such as emissions from vehicles.
Acknowledgments
This work is partly supported by the Grant-In-Aid for Scientific Research (KAKENHI 19H04240) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
-
De Haan, D.O., Corrigan, A.L., Smith, K.W., Stroik, D.R., Turley, J.J., Lee, F.E., Tolbert, M.A., Jimenez, J.L., Cordova, K.E., Ferrell, G.R. (2009) Secondary Organic Aerosol-Forming Reactions of Glyoxal with Amino Acids. Environmental Science and Technology 43(8), 2818-2824
[https://doi.org/10.1021/es803534f]

-
Dou, J., Lin, P., Kuang, B.Y., Yu, J.Z. (2015) Reactive Oxygen Species Production Mediated by Humic-Like Substances in Atmospheric Aerosols: Enhancement Effects by Pyridine, Imidazole, and Their Derivatives. Environmental Science and Technology 49(11), 6457-6465.
[https://doi.org/10.1021/es5059378]

-
Feng, Y., Ramanathan, V., Kotamarthi, V.R. (2013) Brown Carbon: A Significant Atmospheric Absorber of Solar Radiation? Atmospheric Chemistry and Physics 13(17), 8607-8621
[https://doi.org/10.5194/acp-13-8607-2013]

-
Hamilton, J.F., Baeza-Romero, M.T., Finessi, E., Rickard, A.R., Healy, R.M., Peppe, S., Adams, T.J., Daniels, M.J.S., Ball, S.M., Goodall, I.C.A., Monks, P.S., Borrás, E., Muñoz, A. (2013) Online and offline Mass Spectrometric Study of The Impact of Oxidation and Ageing on Glyoxal Chemistry and Uptake onto Ammonium Sulfate Aerosols. Faraday Discussions 165, 447-472
[https://doi.org/10.1039/c3fd00051f]

-
Huang, M., Xu, J., Cai, S., Liu, X., Zhao, W., Hu, C., Gu, X., Fang, L., Zhang, W. (2018) Characterization of Brown Carbon Constituents of Benzene Secondary Organic Aerosol Aged with Ammonia. Journal of Atmospheric Chemistry 75(2), 205-218
[https://doi.org/10.1007/s10874-017-9372-x]

-
Jiang, H., Frie, A.L., Lavi, A., Chen, J.Y., Zhang, H., Bahreini, R., Lin, Y.-H. (2019) Brown Carbon Formation form Nighttime Chemistry of Unsaturated Heterocyclic Volatile Organic Compounds. Environmental Science and Technology Letters 6(3), 184-190
[https://doi.org/10.1021/acs.estlett.9b00017]

-
Kampf, C.J., Jakob, R., Hoffmann, T. (2012) Identification and Characterization of Aging Products in The Glyoxal/ Ammonium Sulfate System-Implications for Light-Absorbing Material in Atmospheric Aerosols. Atmospheric Chemistry and Physics 12(14), 6323-6333
[https://doi.org/10.5194/acp-12-6323-2012]

-
Kaneyasu, N., Yamamoto, S., Sato, K., Takami, A., Hayashi, M., Hara, K., Kawamoto, K., Okuda, T., Hatakeyama, S. (2014) Impact of long-Range Transport of Aerosols on The PM2.5 Composition at A Major Metropolitan Area in The Northern Kyushu Area of Japan. Atmospheric Environment 97, 416-425
[https://doi.org/10.1016/j.atmosenv.2014.01.029]

-
Laskin, A., Smith, J.S., Laskin, J. (2009) Molecular Characterization of Nitrogen-Containing Organic Compounds in Biomass Burning Aerosols Using High-Resolution Mass Spectrometry. Environmental Science and Technology 43(10), 3764-3771
[https://doi.org/10.1021/es803456n]

-
Laskin, A., Laskin, J., Nizkorodov, S.A. (2015) Chemistry of Atmospheric Brown Carbon. Chemical Reviews 115(10), 4335-4382
[https://doi.org/10.1021/cr5006167]

-
Lin, P., Laskin, J., Nizkorodov, S.A., Laskin, A. (2015) Revealing Brown Carbon Chromophores Produced in Reactions of Methylglyoxal with Ammonium Sulfate. Environmental Science and Technology 49(24), 14257-14266
[https://doi.org/10.1021/acs.est.5b03608]

-
Lukács, H., Gelencsér, A., Hammer, S., Puxbaum, H., Pio, C., Legrand, M., Kasper-Giebl, A., Handler, M., Limbeck, A., Simpson, D., Preunkert, S. (2007) Seasonal Trends and Possible Sources of Brown Carbon Based on 2-Year Aerosol Measurements at Six Sites in Europe. Journal of Geophysical Research Atmospheres 112(D23), D23S18
[https://doi.org/10.1029/2006JD008151]

-
Moon, J.-K., Shibamoto, T. (2011) Formation of Carcinogenic 4(5)-Methylimidazole in Maillard Reaction Systems. Journal of Agricultural and Food Chemistry 59(2), 615-618
[https://doi.org/10.1021/jf104098a]

-
Morita, T., Uneyama, C. (2016) Genotoxicity Assessment of 4-Methylimidazole: Regulatory Perspectives. Genes and Environment 38, 38:20
[https://doi.org/10.1186/s41021-016-0050-z]

-
Nakayama, T., Matsumi, Y., Sato, K., Imamura, T., Yamazaki, A., Uchiyama, A. (2010) Laboratory Studies on Optical Properties of Secondary Organic Aerosols Generated During The Photooxidation of Toluene and The Ozonolysis of α-pinene. Journal of Geophysical Research Atmospheres 115(D24), D24204
[https://doi.org/10.1029/2010JD014387]

-
Osada, K., Saito, S., Tsurumaru, H., Hoshi, J. (2019) Vehicular Exhaust Contributions to High NH3 and PM2.5 Concentrations During Winter in Tokyo, Japan. Atmospheric Environment. 206, 218-224
[https://doi.org/10.1016/j.atmosenv.2019.03.008]

-
Pflaum, T., Hausler, T., Baumung, C., Ackermann, S., Kuballa, T., Rehm, J., Lachenmeier, D.W. (2016) Carcinogenic Compounds in Alcoholic Beverages: An Update. Archives of Toxicology 90(10), 2349-2367
[https://doi.org/10.1007/s00204-016-1770-3]

-
Saleh, R., Hennigan, C.J., McMeeking, G.R., Chuang, W.K., Robinson, E.S., Coe, H., Donahue, N.M., Robinson, A.L. (2013) Absorptivity of Brown Carbon in Fresh and Photo-Chemically Aged Biomass-Burning Emissions. Atmospheric Chemistry and Physics 13(15), 7683-7693
[https://doi.org/10.5194/acp-13-7683-2013]

-
Teich, M., van Pinxteren, D., Kecorius, S., Wang, Z., Herrmann, H. (2016) First Quantification of Imidazoles in Ambient Aerosol Particles: Potential Photosensitizers, Brown Carbon Constituents, and Hazardous Components. Environmental Science and Technology 50(3), 1166-1173
[https://doi.org/10.1021/acs.est.5b05474]

-
Tomiyama, H., Tanabe, K., Chatani, S., Kobayashi, S., Fujitani, Y., Furuyama, A., Sato, K., Fushimi, A., Kondo, Y., Sugata, S., Morino, Y., Hayasaki, M., Oguma, H., Ide, R., Kusaka, H., Takami, A. (2017) Observation for Temporal Open Burning Frequency and Estimation for Daily Emissions Caused by Open Burning of Rice Residue. Journal of Japan Society for Atmospheric Environment 52(4), 105-117, [in Japanese]
[https://doi.org/10.11298/taiki.52.105]

-
Updyke, K.M., Nguyen, T.B., Nizkorodov, S.A. (2012) Formation of Brown Carbon Via Reactions of Ammonia with Secondary Organic Aerosols form Biogenic and Anthropogenic Precursors. Atmospheric Environment 63, 22-31
[https://doi.org/10.1016/j.atmosenv.2012.09.012]

-
Vollmuth, T.A. (2018) Caramel Color Safety-An Update. Food and Chemical Toxicology 111, 578-596
[https://doi.org/10.1016/j.fct.2017.12.004]

-
Wu, C., Wang, L., Li, H., Yu, S. (2019) Enhancement of Liquid Chromatography-Ion Trap Mass Spectrometry Analysis of 4(5)-Methylimidazole in Biscuits Through Derivatization with Dansyl Chloride. Journal of Chromatography A 1596 (5), 1-7
[https://doi.org/10.1016/j.chroma.2019.02.061]

-
Yamaguchi, H., Masuda, T. (2011) Determination of 4(5)-Methylimidazole in Soy Sauce and Other Foods by LC-MS/MS after Solid-Phase Extraction. Journal of Agricultural and Food Chemistry 59(18), 9770-9775
[https://doi.org/10.1021/jf201736c]

