Development of 100 μmol mol-1 Cl2 Standard Gas Mixture for Accurate Calibration of Ambient Cl2 Sensors and Stack Emission Monitors
Copyright © 2020 by Asian Association for Atmospheric Environment
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
To accurately measure Cl2 mixing ratio using stack emission monitors and leak monitoring sensors in a workplace, it is essential calibrating monitors and sensors periodically using a Cl2 standard gas mixture. Because there is no reliable analytical technique for Cl2, an international standard for high-pressure Cl2 gas mixtures has not been established. A novel approach for the quantification of Cl2 was developed using quadrupole mass spectrometry (QMS) with an expanded uncertainty of 0.7 μmol mol-1. A 100 μmol mol-1 chlorine (Cl2)/N2 gas mixture was produced through a two-step procedure involving dilution of Cl2 with N2 in high-pressure aluminum cylinders. To check the consistency between 100 μmol mol-1 Cl2 gas mixtures in different cylinders, Cl2 gas mixtures were quantified using the QMS based approach. It was found that four cylinders of 100 μmol mol-1 Cl2 gas mixtures prepared in 2016 agreed within 0.7 μmol mol-1. The long-term stability of 100 μmol mol-1 Cl2 gas mixtures was assessed through changes in the Cl2 mixing ratio over a one-year. It was found that the 100 μmol mol-1 Cl2 gas mixture was stable within ±0.7 μmol mol-1 over one year. Finally, 100 μmol mol-1 Cl2/N2 gas mixture was successfully developed in a high pressure cylinder with an expanded uncertainty of 2.0 μmol mol-1 (k=2; 95% confidence level).
Keywords:
Cl2, Standard gas mixture, Quadrupole mass spectrometry, Stability, Uncertainty1. INTRODUCTION
Chlorine (Cl2) is an extremely reactive and strongly oxidizing agent widely used in the pharmaceutical, plastics, agrochemical, water-treatment, textile, cleaning, and semiconductor industries (Saroha, 2006). Cl2 gas forms HCl upon contact with atmospheric water vapor, attacking the human respiratory system, eyes, and skin. The US National Institute for Occupational Safety and Health recommends an exposure limit of 0.5 ppm in air for 15 minutes (NIOSH, 2007), requiring continuous monitoring of atmospheric Cl2 concentrations in workplaces where the gas is used.
Cl2 concentrations can be determined by ion chromatography after dissolution in water (U.S. EPA, 1996) with continuous monitoring being possible with a gas/liquid collector coupled to an ion-chromatography system (Jung et al., 2019). Optical techniques are not applicable because Cl2 lacks a strong absorption band in the infrared and visible spectral regions. Electrochemical methods can be employed but are prone to interference by other gases and moisture (Menne and Weppner, 1992), although they are widely used because the more accurate ion-chromatography technique does not lend itself to portability. Thus, periodic calibration of electrochemical sensors with standard Cl2 gas mixtures is necessary for Cl2 monitoring to be reliable.
A 100 μmol mol-1 HCl/N2 primary-standard gas mixture in a high-pressure cylinder was recently developed by KRISS with an expanded uncertainty of 1.65 μmol mol-1 (k=2; 95% confidence) ( Jung et al., 2019). For the development of HCl/N2 primary-standard gas mixture, the HCl gas mixtures in the different cylinders were verified using Fourier transform infrared spectroscopy (FTIR). The long-term stability of HCl/N2 primary-standard gas mixture was evaluated using the FTIR. Because there is no reliable analytical technique for Cl2, an international standard for high-pressure Cl2 gas mixtures has not been established.
The objective of this study is development of Cl2 reference gas mixtures which can be used to calibrate various electrochemical sensors and stack emission monitors. To develop primary Cl2 reference gas mixtures, it is essential to verify prepared Cl2 gas mixtures with reliable analytical technique with very low analytical uncertainty. In this study, quantification of Cl2 concentrations using a quadrupole mass spectrometer (QMS) has been developed. This study also describes the development of 100 μmol mol-1 Cl2/N2 standard gas mixtures in high-pressure aluminum cylinders by checking the consistency of gas mixtures between cylinders and their long-term stability.
2. METHODS AND MATERIALS
2. 1 Preparation of Cl2/N2 Gas Mixtures
The Cl2/N2 (source/balance) gas mixtures were produced by a previously reported gravimetric method (ISO 6142-1, 2015), with a custom-designed gas-filling system. A sulfinert-treated manifold and valves were used in the gas-filling system to minimize adsorption loss of Cl2 inside the system. Aluminum cylinders of 10 L volume (Luxfer, Manchester, UK) were used for the preparation of Cl2/N2 gas mixtures. The cylinders were heated at 50-60°C under vacuum condition at 5×10-6 Torr to remove impurities inside a cylinder. The weight of cylinders was measured before and after filling source and balance gases using an automated balance (XP26 003L, Mettler-Toledo, Switzerland) having 26 kg capacity and 1 mg resolution (Kim et al., 2018).
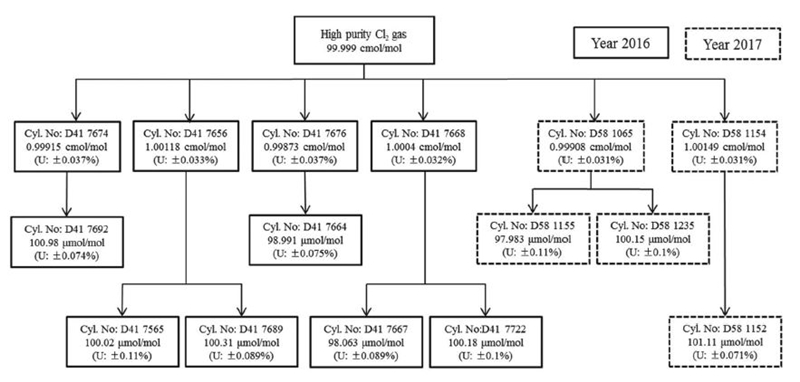
The purity of the Cl2 gas was reported as 99.9995%. Impurities were quantified by the manufacturer (Paik Kwang Industrial, RoK) by gas chromatography with a pulsed-discharge helium-ionization detector, and included O2, N2, and H2O at concentrations of 0.1 μmol mol-1, 0.04 μmol mol-1, and 0.41 μmol mol-1, respectively. High-purity N2 (99.9999%±0.00005% purity; Deokyang, RoK) balance gas was used, with any impurities being quantified by gas chromatography with flame-ionization and thermal-conductivity detectors ( Jung et al., 2019). The 100 μmol mol-1 Cl2/N2 gas mixtures were prepared by two-step dilution of Cl2 with N2, as shown schematically in Fig. 1. Four cylinders of ~1 cmol/mol and six of ~100 μmol mol-1 Cl2 gas mixtures were prepared in 2016. A further two cylinders of ~1 cmol/mol and three of ~100 μmol mol-1 Cl2 gas mixtures were prepared in 2017 (Fig. 1). Expanded relative uncertainties in the 100 μmol mol-1 mixtures ranged from 0.07% to 0.11% with an arithmetic mean of 0.09% (Fig. 1).
2. 2 Cl2 Analysis
To check the consistency between gas cylinders, Cl2 mixing ratios were determined by a QMS (Magic-300, Bongil, Korea), with specifications as summarized in Table 1 and the system as illustrated in Fig. 2 (comprising systems for gas injection, flow control, and analysis). A multi-position valve was used to inject gas into up to five cylinders sequentially, while preventing reactions of Cl2 in the regulator and gas lines with air moisture.
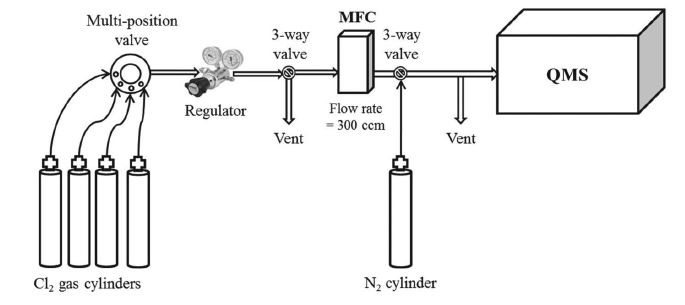
Schematic diagram of the quadrupole mass spectrometer (QMS) Cl2 measurement system. MFC=mass flow controller.
A gas regulator between the injection and flow-control systems controlled gas pressure. The gas line and regulator were flushed three times with new Cl2/N2 gas mixture between cylinder fillings. The gas-mixture flow rate was maintained at 300 mL min-1 using a mass-flow controller. A small fraction of the gas mixture was injected into the QMS system, while the remainder was vented. Pure N2 gas was injected into the QMS system through a three-way valve while the next cylinder was injected through the multi-position valve as shown in Fig. 2.
3. RESULTS AND DISCUSSION
3. 1 The QMS Cl2 Measurement Technique
The QMS ionization chamber ionizes Cl2 to Cl2+, some of which fragments to Cl+Cl+. Chlorine has two isotopes, 35Cl and 37Cl, with 35Cl making up 76% of natural chlorine and 37Cl 24% (Hoering et al., 1961). The mass spectrum of Cl+ should thus display peaks at m/z=35 and 37, whereas Cl2+ displays peaks at m/z=70, 72, and 74. Signals should be relatively high for m/z=37 and 70.
QMS mass spectra of pure N2 and 100 μmol mol-1 Cl2/N2 gas mixtures are shown in Fig. 3, with relatively high intensities being apparent at m/z=35, 36, 37, 38, 40, 70, 72, and 74 for the gas mixture compared with pure N2. The m/z=35 and 37 peaks of the gas mixture are due to Cl+ fragmentation from Cl2+, whereas the m/z=70, 72, and 74 peaks (Fig. 3a) are attributed to Cl2+. The m/z=35 and 70 peaks were therefore used in QMS analysis of gas mixtures.
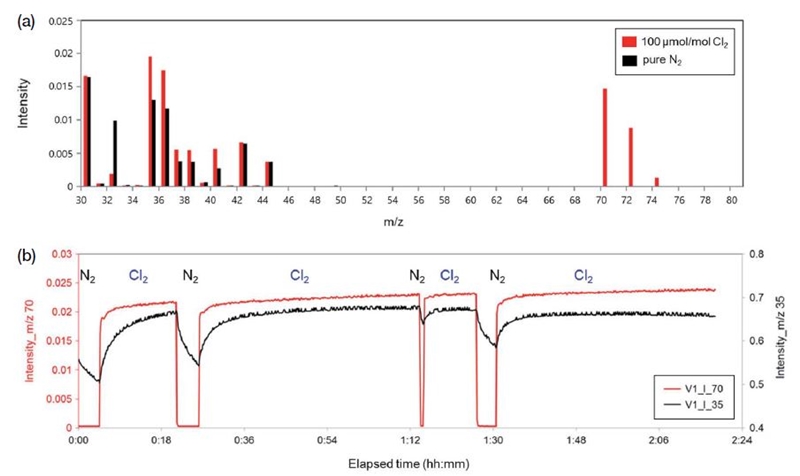
(a) QMS mass spectra of N2 and 100 μmol mol-1 Cl2/N2 gas mixture; (b) temporal variations in m/z=35 and 70 intensities during sequential injections of N2 and Cl2 gas mixture.
Temporal variations in m/z=35 and 70 intensities during sequential injections of gas mixture and N2 are shown in Fig. 3b, with decreases in intensity at m/z=35 and 70 occurring after injection of N2. Even though decrease in intensity at m/z=35 was observed after injection of N2, the intensity at m/z=35 decreased gradually and didn’t reach to zero value whereas significant decrease in intensity at m/z=70 down to zero value was observed after injection of N2. These results (Fig. 3b) indicate that the m/z=70 signal is more suitable for quantification of Cl2 gas mixtures.
The Cl2/N2 mixing ratio was determined after calibrating the QMS system with the 100 μmol mol-1 Cl2/N2 gas mixture (Fig. 4). The QMS Cl2 response did not stabilize until >1 h after injection of the gas mixture (Fig. 4a), so a correction method was introduced to correct drifts in Cl2 response with time (Fig. 4b). Reference and sample cylinders were injected sequentially over the same time intervals (Fig. 4b), with N2 being injected between reference and sample cylinders to clean gas lines and the QMS system.
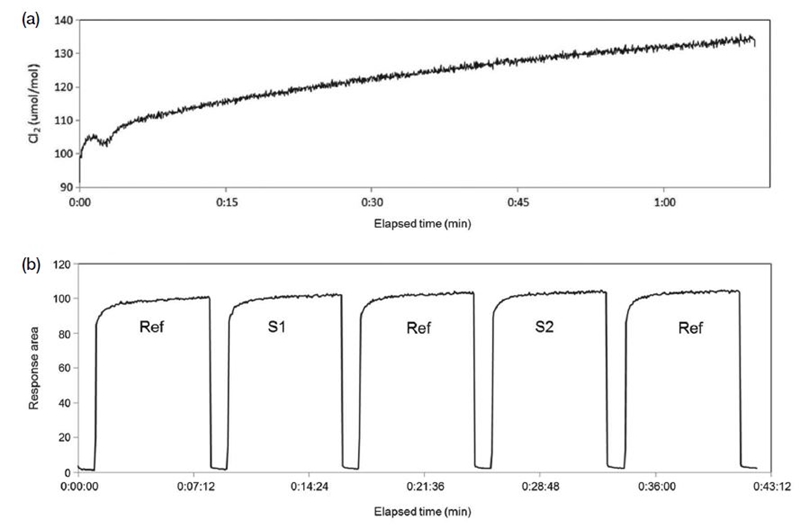
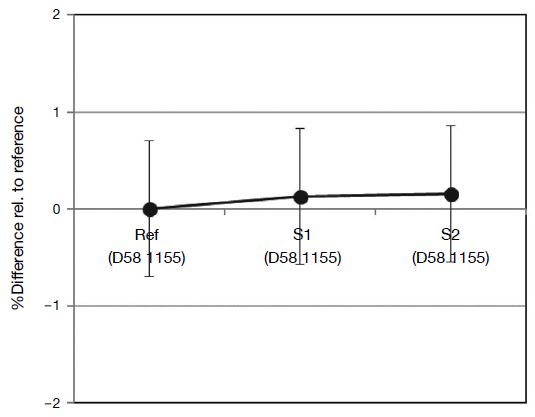
(a) Temporal variation in gas-mixing ratio; (b) variations during sequential injection of reference and sample gas mixtures. The reference Cl2 gas mixture (D58 1155) was used as samples S1 and S2.
Measurement sensitivity was defined as the ratio of QMS Cl2 response to gravimetric Cl2 value:
| (1) |
The percentage difference between sample and reference cylinders was calculated as follows:
| (2) |
The validity of this comparison method was checked by injecting 100 μmol mol-1 Cl2/N2 gas mixture continuously for 7 min, followed by 1 min N2 injection, with reference cylinder D58 1155 being injected as “samples” 1 and 2 (Figs. 4 and 5). The three Cl2 values agreed within 0.7%, indicating that the Cl2 comparison method is valid for the 100 μmol mol-1 Cl2/N2 gas mixture with an expanded uncertainty of 0.7 μmol mol-1.
3. 2 Verification and Stability of Gas Mixtures
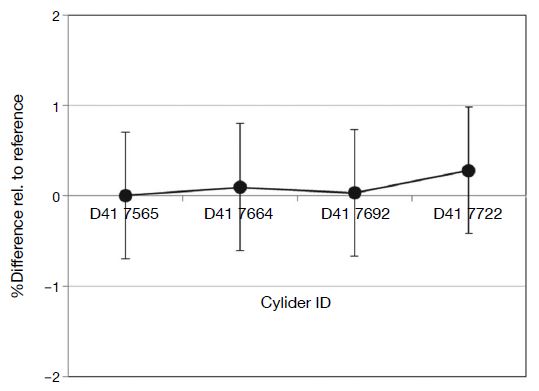
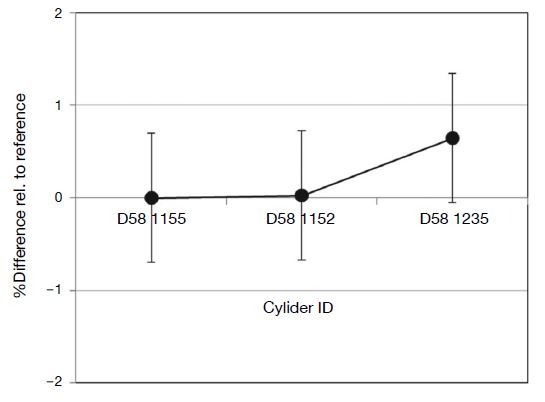
The 100 μmol mol-1 Cl2/N2 gas mixtures prepared in 2016 were analyzed using the analytical procedure illustrated in Fig. 4, with cylinder D41 7565 as a working reference cylinder. Results are summarized in Fig. 6. Analytical results of the four cylinders agreed within 0.7 μmol mol-1. This was repeated with gas mixtures prepared in 2017 (Fig. 7), with results again agreeing within 0.7 μmol mol-1.
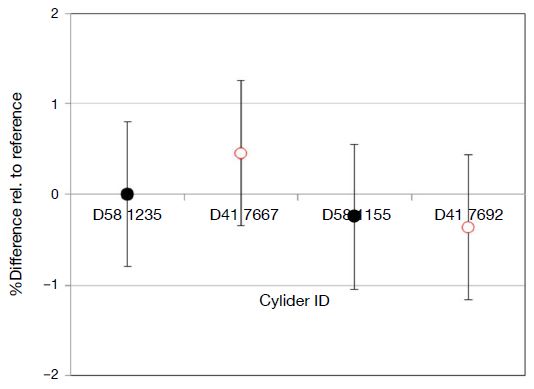
The long-term stability of the high-pressure gas mixtures was assessed through changes in the Cl2 mixing ratio. Gas mixtures D41 7667 and 7692 (2016), and D58 1235 and 1155 (2017), were analyzed by QMS with D58 1235 as a working reference cylinder. Results are shown in Fig. 8. No significant changes in gas compositions were observed (Fig. 8), with the four cylinders being consistent within the analytical uncertainty of 0.7 μmol mol-1. The gas mixtures can therefore be assumed to be stable within ±0.7 μmol mol-1over one year.
3. 3 Uncertainty Estimation
The main uncertainties of the gas mixture were gravimetric preparation of gas mixture in high-pressure cylinder, verification within different cylinders, and long-term stability. The preparation uncertainty of the 100 μmol mol-1 Cl2/N2 gas mixture was determined to be 0.09 μmol mol-1, whereas the verification uncertainty was 0.7 μmol mol-1 (Figs. 6 and 7), and the uncertainty related to stability was 0.7 μmol mol-1 (Fig. 8). The standard uncertainty (uCRM) of a certified reference material (CRM) was calculated as follows:
| (3) |
where uprep, uver, and ustb represent standard uncertainty related to gravimetric preparation, verification between different cylinders, and stability within cylinder, respectively. The standard uncertainty for the 100 μmol mol-1 Cl2/N2 standard gas mixture was determined to be 1.0 μmol mol-1 (Table 2). Thus, the 100 μmol mol-1 Cl2/N2 standard gas mixture was successfully developed in a high-pressure cylinder with an expanded uncertainty of 2.0 μmol mol-1 (k=2; 95% confidence level).
4. CONCLUSION
The QMS can successfully be applied in determining the consistency and stability of high-pressure 100 μmol mol-1 Cl2/N2 standard gas mixtures. Gas cylinders prepared in 2016 and 2017 were successfully verified within an uncertainty of 0.7 μmol mol-1, with the mixtures being stable within ±0.7 μmol mol-1 over a one-year period. The expanded uncertainty of 100 μmol mol-1 Cl2/N2 gas mixtures is 2.0 μmol mol-1 (k=2; 95% confidence level). This Cl2 standard gas mixture can contribute to improve the accuracy of stack emission monitors and leak monitoring sensors in a workplace.
Acknowledgments
This work was supported by a grant (20011031) from the Korean Research Institute of Standards and Science (KRISS) under the Basic R&D Project of Establishment of National Gas Analysis Measurement Standards and Improvements of Calibration and Measurement Capabilities.
References
-
Hoering, T.C., Parker, P.L. (1961) The geochemistry of the stable isotopes of chlorine. Geochimica et Cosmochimica Acta, 23(3-4), 186-199.
[https://doi.org/10.1016/0016-7037(61)90043-6]

- International Organization for Standardization (ISO) (2015) Gas analysis-preparation of calibration gas mixtures-gravimetric methods for class. International Organization for Standardization (ISO 6142-1).
-
Jung, J., Kim, B., Oh, S. (2019) Development of 100 μmol mol-1 HCl primary standard gas mixtures in high-pressure cylinders. Metrologia, 56(1), 015007.
[https://doi.org/10.1088/1681-7575/aaf2b1]

-
Kim, M.E., Kang, J.H., Kim, Y.D., Lee, D.S., Lee, S. (2018) Development of accurate dimethyl sulphide primary standard gas mixtures at low nanomole per mole levels in high-pressure aluminum cylinders for ambient measurements. Metrologia, 55(2), 158-166.
[https://doi.org/10.1088/1681-7575/aaa583]

-
Menne, A., Weppner, W. (1992) Influence of moisture at solid/gas interfaces in electrochemical Cl2 sensor. Sensors and Actuatuators B: Chemical, 9(1), 79-82.
[https://doi.org/10.1016/0925-4005(92)80197-6]

- National Institute for Occupational Safety and Health (NIOSH) (2007) NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 2005-149. https://www.cdc.gov/niosh/docs/2005-149/pdfs/2005-149.pdf
-
Saroha, A.K. (2006) Safe handling of chlorine. Journal of Chemical Health and Safety, 13(2), 5-11.
[https://doi.org/10.1016/j.chs.2005.02.005]

- U.S. Environmental Protection Agency (U.S. EPA) (1996) Determination of chloride from HCl/Cl2 emission sampling train by anion chromatography. Method 9057, U.S. EPA. https://19january2017snapshot.epa.gov/sites/production/files/2015-12/documents/9057.pdf