A Comparison between Wet-only and Bulk Deposition at Two Forest Sites in Japan
Abstract
To investigate the effects of forest and the surrounding natural and anthropogenic sources on the bulk depositions on forested land, this study examined differences in ion concentrations between wet-only and bulk samples at two forested sites in Japan. The surrounding natural and anthropogenic sources at each site were different; Shirasaka is in a rural area and Tanashi is an urban environment. The volume weighted (vw) mean concentrations of K+ and Ca2+ in the bulk samples were significantly (p<0.05) higher than those in the wet-only samples at both sites. The forest canopy and a nearby incineration plant were hypothesized to be the main sources of K+ contaminants at Shirasaka and Tanashi, respectively. The transport of sea salt and urban dust may explain the presence of enriched Ca2+ concentrations in the bulk samples at Shirasaka and Tanashi, respectively. The NH4+ concentrations in the Shirasaka bulk samples were significantly (p<0.05) lower than those in the wet-only samples. The vw mean SO42- and NO3- concentrations in both sample types were not significantly different at either site. This study demonstrated that the ion concentration differences between wet-only and bulk samples were affected by nearby natural and anthropogenic sources even at forest sites, similar to previous findings for non-forested locations. However, the K+ concentration differences between wet-only and bulk samples may be higher owing to forest sources, even in the absence of anthropogenic sources.
Keywords:
Atmospheric deposition, Dry deposition, Forests, Urban areas, Rural areas1. INTRODUCTION
Atmospheric deposition onto the forest canopy is a key nutrient cycling process in forest ecosystems (Lindberg et al., 1986; Swank, 1984). As atmospheric deposition comprises both wet and dry depositions, the two components must be quantified to understand nutrient cycling in forests. Wet deposition (hereafter referred to as wet-only deposition) is collected using a wet-only sampler, which prevents dry deposition contamination with precipitation (Chantara and Chunsuk, 2008; Dämmgen et al., 2005; Staelens et al., 2005). Bulk deposition, collected by a bulk sampler, has been used as a surrogate for wet-only deposition, particularly at forest sites (e.g., Imamura et al., 2012). The bulk sampler is much easier to operate than the wet-only sampler and, unlike the wet-only sampler, it does not require an electric power supply, allowing a stable long-term operation even at remote forest sites. However, a bulk sampler will eventually become contaminated with gas and dry particle depositions, which may result in large uncertainties in atmospheric deposition measurements (Dämmgen et al., 2005).
Previous studies have compared wet-only and bulk depositions at non-forested locations and have shown a variety of contaminants in the bulk deposition. For example, Ca2+, Mg2+, and K+ concentrations are frequently higher in bulk deposition than in wet-only deposition owing to soil-derived and sea salt particle contamination (Thimonier, 1998; Stedman et al., 1990; Table 1). Additionally, NO3-, SO42-, and NH4+ concentrations are occasionally higher in the bulk deposition than the concentrations in the wet-only deposition owing to local or regional industrial plant emissions (Stedman et al., 1990; Table 1).

The ratio of vw mean wet-only and bulk ion concentrations in precipitation, measured at several locations around the world. Also provided are reference, and sampling intervals (SI).
However, at forested sites there is little information on the differences between wet-only and bulk depositions (Balestrini et al., 2007; Chiwa et al., 2007; Staelens et al., 2005; Richter and Lindberg, 1988; Table 1). One study showed that the bulk deposition was largely affected by K+-containing aerosols, emitted from the nearby forest canopy (e.g., pollen; Likens et al., 1994). The surrounding natural and anthropogenic sources, such as volcanoes, deserts, marine environments, agricultural activities, automobile traffic, and industrial plants, may also affect bulk deposition at forested sites. Additional examinations of the effects of forest and its surrounding natural and anthropogenic sources on the bulk deposition are requisite, particularly at forested sites.
To investigate the effects of forest and its surrounding natural and anthropogenic sources on the bulk depositions on forested land, a year-long wet-only and bulk sampling at two forest sites with different natural and anthropogenic sources was conducted in this study. The difference between the two components was examined with the result potentially validating the use of a bulk sampler as a surrogate for a wet-only sampler to measure atmospheric deposition on forested land.
2. MATERIALS AND METHODS
2. 1 Site Description
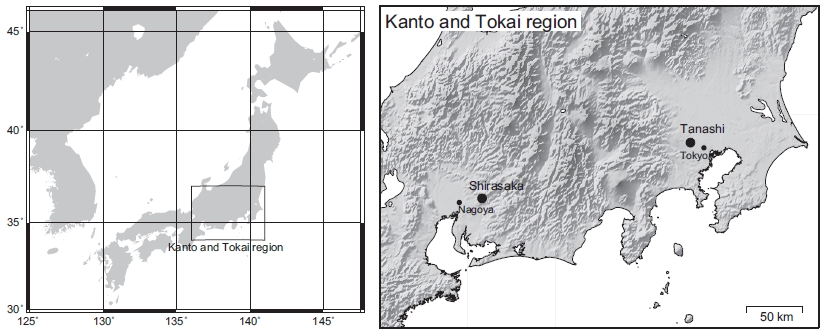
We selected two forest sites with different degrees of urbanization. The rural forest site, Shirasaka, is owned by the Ecohydrology Research Institute and is a part of The University of Tokyo Forests (35°22ʹN, 137°16ʹE, 303.6 m a.s.l.) in Aichi Prefecture, Japan (Fig. 1). Shirasaka is situated 23.9 km from the center of Nagoya City (about 6,900 people per km2) and the nearby traffic density is about 4,000 vehicles per day (Seto City, 2013). Ise Bay, the closest sea, is approximately 40 km southwest of the site. Shirasaka is underlain by weathered granitic rocks with cambisols and is covered by a mixed broadleaf-conifer forest composed of Quercus serrata, Pinus densiflora, and evergreen broadleaved trees.
The urban forest site, Tanashi, is owned by The University of Tokyo Tanashi Forest (35°74ʹN, 139°54ʹE, 60 m a.s.l.) and is located within the Tokyo metropolitan area in the Kanto region. The site is situated approximately 15 km from the center of Tokyo (about 14,400 people per km2; Fig. 1) and traffic density is about 70,000 vehicles per day near the site (Ministry of Land, Infrastructure, Transport, and Tourism, Japan, 2005). Tokyo Bay, the closest sea, is about 30 km to the southeast and an incineration plant exists at approximately 3 km south of the site. This site is an isolated forest (9.1 ha) within a residential area adjacent to an experimental farm (22.2 ha). The soils are andisols (i.e., volcanic ash) and the demonstration forest is composed of 244 tree species.
2. 2 Sample Collection
Wet-only precipitation was collected using automatic wet-only samplers (Ogasawara-Keiki, US-410) with 20 cm Teflon orifice diameters at heights of approximately 100 cm, located in the open meteorological stations at both sites. The meteorological stations at both sites are more than 30 m from the forest. The bulk deposition was measured with a bulk sampler at the same stations. At Shirasaka, the bulk sampler was constructed using a 24 cm polyethylene funnel attached to a 50 L polyethylene bottle, with glass wool placed in the funnel to prevent large particle contamination. The height of the funnel was approximately 70 cm. At Tanashi, the bulk sampler was constructed using a 21 cm polyethylene funnel attached to a 10 L polyethylene bottle, with a nylon mesh placed in the funnel. The polyethylene bottle was wrapped in aluminum foil to reflect sunlight and the height of the funnel was approximately 60 cm. The small difference in funnel diameters did not affect the measurements because the sampling area only has a minor influence on rainfall quantification precision (Thimonier, 1998). At Shirasaka, 12 sets of wet-only and bulk depositions, which averaged one sample each month, were sampled between June 19, 2012 and June 21, 2013. At Tanashi, 16 sets of wet-only and bulk depositions, averaging one sample every 4 weeks, were sampled between February 16, 2011 and February 2, 2012. For each sample, a new plastic bag was rinsed with deionized water and placed inside the polyethylene bottle, and the funnel was washed with deionized water to prevent contamination. Additionally, the glass wool was replaced and the nylon mesh was washed. Wind speed and direction were measured at a meteorological station at Shirasaka and atop a 26 m meteorological tower at Tanashi. The daily wind direction was calculated by a unit-vector average.
2. 3 Chemical Analysis and Quality Control
The pH and electrical conductivity of the water samples were measured after field sampling with a HORIBA D-54 at Shirasaka and with a Toa-DKK WM-32EP at Tanashi. At Shirasaka, the water samples were transferred to acid-washed polyethylene bottles and brought to the laboratory. After filtering through a 0.2-μm membrane (Advantec, 13CP020AS), anion (i.e., Cl-, SO42-, and NO3-) concentrations between July 19, 2012 and November 20, 2012 were measured using ion chromatography (Shimadzu, LC-10A). Additional anion (i.e., Cl-, SO42-, and NO3-) and NH4+ concentrations between December 18, 2012 and June 21, 2013 were also measured using ion chromatography (Shimadzu, HIC-6A). After filtering through a 0.45-μm membrane (Advantec, 25CS045AS), cation (i.e., Na+, K+, Mg2+, and Ca2+) concentrations during all periods were measured by flame emission spectrometry (Hitachi, Z-2310). At Tanashi, the water samples were transferred to clean polyethylene bottles and brought to the laboratory. Then, the samples were filtered through a 0.2-μm membrane (Minisarut, RC15), and anion (i.e., Cl-, SO42-, and NO3-) and cation (i.e., Na+, K+, Mg2+, Ca2+, and NH4+) concentrations during all periods were measured using ion chromatography (Shimadzu, LC-10A). H+ concentrations were calculated on the basis of the pH measurements.
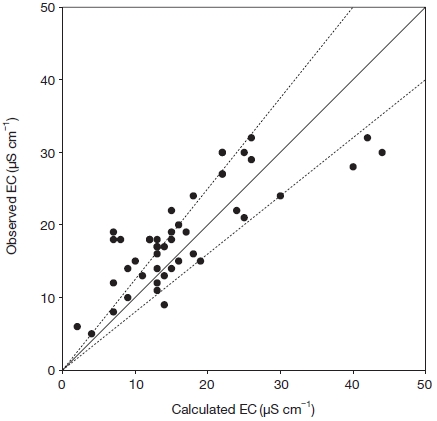
To assure high data quality, ion balances were verified, and the measured and calculated conductivities were compared according to the ICP Forest technical report (EC-UN/ECE, 2000). The data collected between December 18, 2012 and June 21, 2013 at Shirasaka and all sampling data at Tanashi were used in this comparison. When the sum of cation and anion for the individual samples was less than 50, 100, and 500 mmol m-3, the difference between the sum of cation and that of anion was less than 60, 30, and 15%, respectively. Moreover, the difference between measured and calculated conductivities was almost less than 25% (Fig. 2).
2. 4 Data Analysis
The amount of water and the ion concentrations in the wet-only and bulk depositions were compared. First, the annual mean ion concentrations in the wet-only and bulk depositions were calculated as precipitation volume weighted (vw) means. Second, a Wilcoxon signed rank test was used to test for differences in the amount of rainfall and ion concentration between each sample type.
To elucidate the ion sources, the seasonal variations of wet-only and bulk deposition were compared, and principal component analysis (PCA) was performed on bulk sample concentrations (Sakugawa et al., 1993). The Wilcoxon rank test and PCA were performed with R version 2.10 (R Development Core Team, 2009).
3. RESULTS
3. 1 Wind Direction and Speed
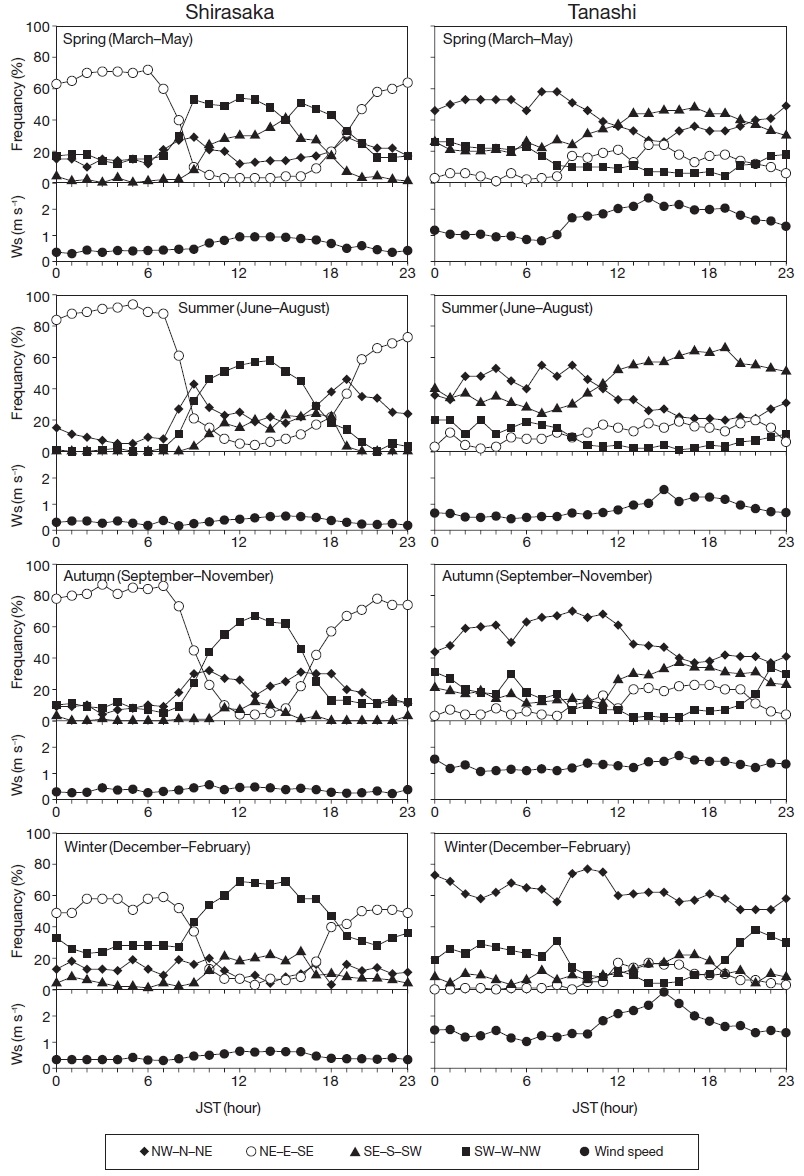
Fig. 3 illustrates the diurnal variations in wind direction and the average hourly wind speed during each season at Shirasaka and Tanashi. At Shirasaka, easterly and westerly winds prevailed during the day and night, respectively, and wind speed was generally lower than that at Tanashi. A strong northerly wind characterized the autumn, winter, and spring seasons at Tanashi, while in the summer, a southerly wind prevailed during the day.
3. 2 Comparison of Wet-only and Bulk depositions
Table 2 summarizes the rainfall, vw means of pH and ion concentrations in wet-only and bulk samples, ratios of the vw mean ion concentrations in bulk to wet-only deposition, and significance levels of the concentration differences between the samples types. At Shirasaka, the vw mean concentrations of Na+, Cl-, K+, Mg2+, and Ca2+ in bulk samples were significantly (p<0.01) higher than those in wet-only samples, with the highest bulk to wet-only deposition ratio found for K+ (3.34), followed by Ca2+ (1.65), Na+ (1.50), Mg2+ (1.37), and Cl- (1.19). The vw mean NH4+ concentrations in the bulk samples were significantly (p<0.05) lower than those in the wet-only samples at Shirasaka.

Rainfall (mm), vw mean pH and ion concentrations (μeq L-1) in wet-only and bulk depositions, ratios of the vw mean concentrations in wet-only to bulk depositions, and Wilcoxon signed rank test results at Shirasaka and Tanashi.
At Tanashi, the vw mean Na+, K+, and Ca2+ concentrations in the bulk samples were significantly (p<0.05) higher than those in the wet-only samples, and high ratios of bulk to wet-only deposition were found for K+ (1.81) and Ca2+ (1.59) concentrations. The vw means of Cl- and H+ concentrations in the bulk samples were both significantly (p<0.05) lower than those in the wet-only samples. The bulk to wet-only deposition ratios for Cl- and H+ concentrations were 0.92 and 0.67, respectively. The vw mean concentrations of SO42- and NO3- in both sample types showed no statistically significant difference at either site.
3. 3 Seasonal Variations in Wet-only and Bulk Depositions
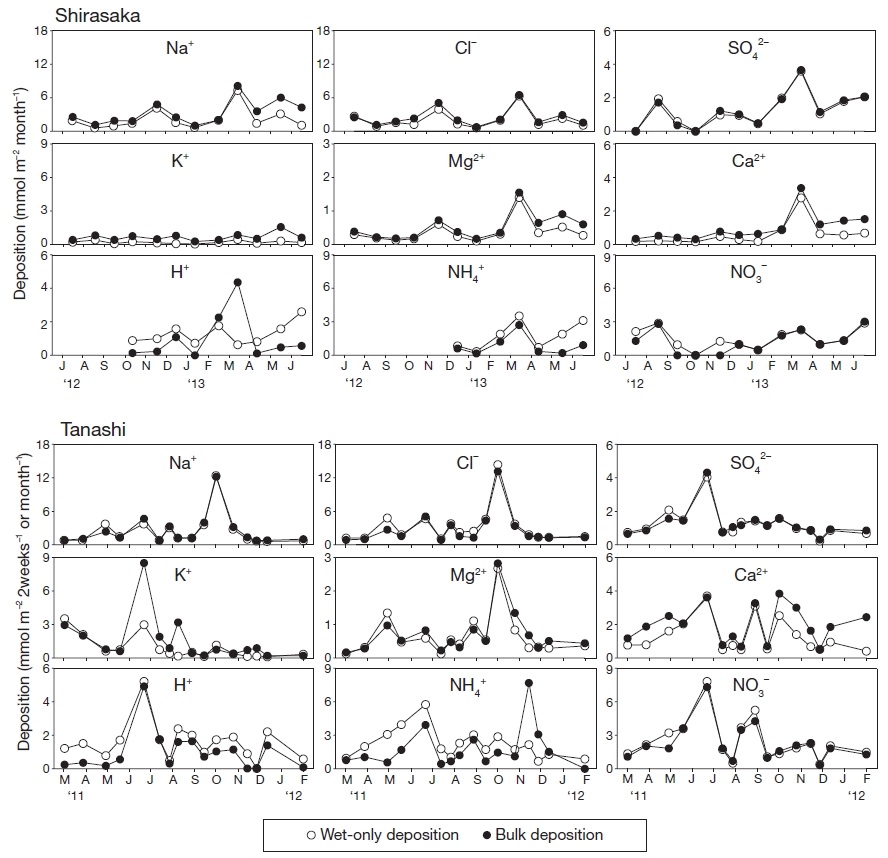
Fig. 4 compares the seasonal variations of wet-only and bulk depositions at each site. For most ions, the seasonal variation in bulk deposition matched the variation in wet-only deposition. The seasonal variation in the bulk Ca2+ deposition was similar to the variation in bulk Na+, Cl-, and Mg2+ deposition at Shirasaka, whereas bulk Ca2+ deposition overwhelmed the wet-only Ca2+ deposition between October and May at Tanashi. Bulk deposition of H+ exceeded H+ wet-only deposition sporadically in February and March at Shirasaka. However, at Tanashi, the H+ bulk deposition was less than H+ wet-only deposition throughout the year. The difference in H+ concentrations between wet-only and bulk depositions increased between October and May; this coincided with the increased differences in Ca2+ deposition at Tanashi. Furthermore, bulk K+ depositions occasionally surpassed the wet-only K+ depositions from June to August at Tanashi.
3. 4 Principal Component Analysis of Bulk Samples
Table 3 shows the results of PCA at Shirasaka and Tanashi. The PCA analyses accounted for 94% and 92% of the total variance at Shirasaka and Tanashi, respectively. At Shirasaka, the first-factor group did not show a major component. However, the minor component was a group of Na+, Mg2+, Ca2+, SO42-, and NH4+. The second-factor group was NO3- and accounted for 11% of the variability in the PCA. The third factor group was K+ and accounted for 10% of the variability in the PCA. At Tanashi, all factor groups showed only minor components. The first-factor group was Na+, Cl-, Mg2+, and SO42- and the second-factor group was H+, NO3-, and K+. The third factor comprised H+, Cl-, and Na+ and NH4+ and Ca2+ groups and the fourth factor was also a group of NH4+ and Ca2+.
4. DISCUSSION
4. 1 Potassium
The vw mean K+ concentration in the bulk samples was 3.34 times higher (p<0.01) than the concentration in the wet-only samples at Shirasaka (Table 2). Non-sea salt K+ constituted more than 83% of the bulk K+ concentration (nss-K+=K+ - 0.0355×Na+; Lai et al., 2007). Therefore, a large portion of bulk K+ concentrations at Shirasaka could not be attributed to a sea source. A strong positive correlation between K+ and Ca2+ concentrations in bulk samples indicates that the increased K+ and Ca2+ concentrations are due to soil (Staelens et al., 2005; Akkoyunlu and Tayanç, 2003) and volcanic ash (Chiwa et al., 2007) contaminants. At Shirasaka, there was only a weak correlation between K+ and Ca2+ concentrations in the bulk samples (r=0.41, Pearson correlation coefficient), thus indicating that the K+ did not originate from the soil. The May peak in bulk sample K+ concentrations (Fig. 4) may relate to the high abundance of plant pollen at that time (Likens et al., 1994). Additionally, PCA showed that K+ was independent source component (Table 3). The enriched K+ concentrations in the bulk samples at Shirasaka were the second highest among those observed in previous studies (Table 1). The highest recorded value was 4.41 (ratio of the vw mean concentrations in wet-only to bulk depositions) at Oak Ridge, which was also located at the opening of an extended mixed deciduous forest (Richter and Lindberg, 1988; Table 1). Gosz (1980) showed that bulk K+ depositions in New Mexico were higher near aspen stands than in areas without stands nearby. Significant quantities of K+ can be transported by aerosols or water droplets from the canopy because deciduous tree species emit large amounts of K+ through leaching processes (Gosz, 1980). Other studies at forested sites inferred that the rich K+ concentrations in bulk depositions could be a result of contamination by K+-containing aerosols from local forest canopy sources (Balestrini et al., 2007; Lovett and Lindberg, 1984). As there are no anthropogenic emission sources around Shirasaka, we conclude that the large difference in K+ concentrations between wet-only and bulk samples at Shirasaka is due to forest canopy aerosol contamination of the bulk samples.
The vw mean K+ concentration in the bulk samples was 1.81 times higher (p<0.05) than the concentration in the wet-only samples at Tanashi (Table 2). Comparing the seasonal variation of wet-only and bulk K+ depositions provides an insight into the contaminant source. The bulk K+ deposition substantially exceeded wet-only K+ deposition at Tanashi, particularly in the summer months (June to September) (Fig. 4) when southerly winds prevailed during the day (Fig. 3). Kamataki et al. (2000, 1995) reported that dry deposition of K+ in the Kanto region was primarily from combustion sources. An incineration plant exists about 3 km to the south of Tanashi and PCA showed that K+ was associated with NO3- and H+ as a minor component (Table 3), indicating that K+ was transported to the site from an industrial source. At Tanashi, no significant correlations between K+ and Ca2+ concentrations were found in the bulk samples (r=- 0.24), thus suggesting non-soil sources of K+. Additionally, non-sea salt K+ constituted more than 81% of the bulk K+ concentration. Unlike Shirasaka, the relationship between wet-only and bulk K+ depositions at Tanashi varied throughout the year (Fig. 4); thus, contaminants, such as ashes or aerosols from nearby plant, transported by southerly winds could explain the sporadic increases in bulk K+ deposition.
4. 2 Calcium
The vw mean Ca2+ concentration in the bulk samples was 1.65 times higher (p<0.001) than that in wet-only samples at Shirasaka (Table 2). The Ca2+ contamination may not be from the soil because no significant correlations were found between K+ and Ca2+ concentrations in the bulk samples (Section 4.1). The other potential source of Ca2+ is sea salt (Stedman et al., 1990). Significant (p<0.01) positive correlations were found between concentrations of Ca2+ and Na+ (r=0.93), Cl- (r=0.81), and Mg2+ (r=0.96) in the bulk samples. These strong correlations are observed from the seasonal bulk deposition patterns for Ca2+, Na+, Cl-, and Mg2+ at Shirasaka (Fig. 4). Additionally, PCA indicated that Ca2+ was derived from a marine source component (i.e., Na+ and Mg2+) (Table 3). These data suggest that Ca2+ was supplied to Shirasaka with ions of marine origin (i.e., Na+, Cl-, and Mg2+).
The vw mean Ca2+ concentration in the bulk samples was 1.59 times higher (p<0.001) than the concentration in wet-only samples at Tanashi (Table 2). Again, the origin of the contaminated Ca2+ may not be the soil (Section 4.1). Unlike those at Shirasaka, bulk sample Ca2+ concentrations exhibited no significant correlation with Na+, Cl-, or Mg2+ concentrations at Tanashi (r=0.09, r=0.20, and r=0.46, respectively), thus suggesting a non-marine source contribution to the Ca2+ contamination. Another possible source of the Ca2+ contamination at Tanashi is urban dust from the city (Lee et al., 1992). A group of Ca2+ and NH4+ was identified as a minor component in the PCA (Table 3). Sakurai et al. (2002) reported that the concentrations of gaseous ammonia increased owing to a nearby anthropogenic source in the Kanto region, which indicates that Ca2+ at Tanashi may have also been transported from a nearby source. In addition, Yokota et al. (2010) reported that road dust was the largest source of Ca2+ in Tokyo. As Tanashi is located near paved roads with heavy traffic (Section 2.1), road and tire wear represent significant sources of Ca2+-containing aerosols. This hypothesis is supported by the timing of the bulk sample Ca2+ concentration deviations from the wet-only sample concentrations (Fig. 4, Section 3.3). These deviations correspond to months with strong winds (Fig. 3) that are capable of transporting large-sized particles to the isolated forest site.
4. 3 Acidity and Ammonium
The vw mean H+ concentration in the bulk samples was 0.67 times lower (p<0.01) than the concentration in the wet-only samples at Tanashi (Table 2). The calculated neutralization factor indicated that Ca2+ was the highest acid-neutralization ion in the Tanashi bulk samples (neutralization factor of 0.83). In addition, bulk deposition of H+ was less than wet-only deposition throughout the year, and these differences increased when dry Ca2+ deposition increased (Fig. 4). The cooccurrence of these observed phenomena was due to the increased acidity reduction by Ca2+ compounds from dry deposition (Lee and Longhurst, 1992). Therefore, the increased input of Ca2+ via dry deposition resulted in a significant reduction of H+ in bulk deposition samples at Tanashi.
The vw mean NH4+ concentration in the bulk samples was 0.50 times lower (p<0.05) than the concentration in the wet-only samples at Shirasaka (Table 2). Low ratios of NH4+ in bulk to wet-only deposition were reported in the United Kingdom by Stedman et al. (1990) and in India by Kulshrestha et al. (1995) (Table 1). Stedman et al. (1990) attributed the NH4+ reduction in the bulk samples to NH4+ volatilization or biological activity. In contrast to these results, the vw mean NH4+ concentration in bulk samples was significantly higher than that in the wet-only samples in Italy (Balestrini et al., 2007; Mosello et al., 1988), Japan (Chiwa et al., 2007; Aikawa et al., 2003), Turkey (Akkoyunlu and Tayanç, 2003), and in Thailand (Chantara and Chunsuk, 2008) (Table 1). These sites may have been affected by nearby gases or other particles. As the local anthropogenic sources at each site differ, a careful examination of the surrounding area at each study site would provide a better understanding of NH4+ deposition.
4. 4 Sulfate and Nitrate
The differences in the vw mean SO42- and NO3- concentrations between the wet-only and bulk samples were not significant at either site (ratios of 0.80-1.07; Table 2). Dry depositions of SO42- and NO3- were produced by particle and gaseous depositions. Hicks (1985) reported that artificial collection surfaces failed to measure gaseous deposition. Therefore, the small difference in SO42- and NO3- between the two sample types could be due to the gaseous deposition capturing capacity of the bulk sampler. These results also indicate that a bulk sampler is acceptable for collecting SO42- and NO3- wet-only samples in an urban forest as long as the surrounding emission levels are similar to those observed at Tanashi.
5. CONCLUSIONS
At the rural forest site, bulk K+ concentrations were affected by forest sources. In contrast, bulk Ca2+ concentrations were affected by nearby anthropogenic sources, a result which agrees with previous studies of non-forested areas. At the urban forest site, bulk K+, Ca2+, and NH4+ concentrations were affected by nearby natural and anthropogenic sources. Thus, the difference between ion concentrations in wet-only and bulk deposition were affected by nearby natural and anthropogenic sources at both forested sites. However, the K+ concentration difference between wet-only and bulk samples may be higher owing to forest sources, even in the absence of anthropogenic sources. Further validation of the effect of forest aerosols on the bulk K+ concentration is required.
Our results quantify the possible errors resulting from the use of a bulk sampler to collect wet-only samples of atmospheric deposition on forested land. However, results from both forest sites indicate that a bulk sampler can substitute for a wet-only sampler for sampling SO42- and NO3-. Sampling method improvements could assure the reliability of these bulk measurements. Currently, bulk ion concentrations are measured at ten forest sites in Japan by universities and seven forest sites by the Forestry and Forest Research Institute. Improving the sampling method will facilitate a greater spatial coverage and allow for the collection of long-term atmospheric deposition measurements using bulk sampler.
Acknowledgments
The authors thank Dr. Masanori Katsuyama of Kyoto University for lending us a wet-only sampler. The authors also thank Mr. Masaki Matsui, Ms. Yukiko Kamata, and Ms. Natsumi Niinomi for their field observations and chemical analyses for the Shirasaka samples, also Dr. Jun Shi, Ms. Chiai Kosaku, Dr. Tomohiro Egusa, and Dr. Tomoki Oda for their support in the field observations at Tanashi.
References
-
Aikawa, M., Hiraki, T., Tamaki, M., Shoga, M., (2003), Difference between filtering-type bulk and wet-only data sets based on site classification, Atmospheric Environment, 37, p2597-2603.
[https://doi.org/10.1016/s1352-2310(03)00214-0]

-
Akkoyunlu, B.O., Tayanç, M., (2003), Analyses of wet and bulk deposition in four different regions of Istanbul, Turkey, Atmospheric Environment, 37, p3571-3579.
[https://doi.org/10.1016/s1352-2310(03)00349-2]

-
Balestrini, R., Arisci, S., Brizzio, M.C., Mosello, R., Rogora, M., Tagliaferri, A., (2007), Dry deposition of particles and canopy exchange: Comparison of wet, bulk and throughfall deposition at five forest sites in Italy, Atmospheric Environment, 41, p745-756.
[https://doi.org/10.1016/j.atmosenv.2006.09.002]

-
Chantara, S., Chunsuk, N., (2008), Comparison of wet-only and bulk deposition at Chiang Mai (Thailand) based on rainwater chemical composition, Atmospheric Environment, 42, p5511-5518.
[https://doi.org/10.1016/j.atmosenv.2008.03.022]

- Chiwa, M., Ide, J., Ohgi, D., Tashiro, N., Koga, S., Shibata, H., Satoh, F., Otsuki, K., (2007), Chemical characteristics of precipitation and streamwater at the Ashoro Research Forest and Kasuya Research Forest, Bulletin of The Kyushu University Forest, 88, p33-43, (in Japanese).
- Dämmgen, U., Erisman, J.W., Cape, J.N., Grünhage, L., Fowler, D., (2005), Practical considerations for addressing uncertainties in monitoring bulk deposition, Environmental Pollution, 134, p535-548.
- EC-UN/ECE, (2000), Intensive Monitoring of Forest Ecosystems in Europe, 2000 Technical Report, EC, UN/ECE 2000, Brussels, Geneva, p1-191.
-
Gosz, J.R., (1980), Nutrient budget studies for forests along an elevational gradient in New Mexico, Ecology, 61, p515-521.
[https://doi.org/10.2307/1937417]

- Hicks, B.B., (1985), Network monitoring of dry deposition of trace gases and submicron particles. In: Special Environmental Report No.16, Lectures presented at the WMO Technical Conference on observation and measurement of atmospheric contaminants, TECOMAC, World Meteorological Organization, Geneva, p387-393.
-
Imamura, N., Tanaka, N., Ohte, N., Yamamoto, H., (2012), Nutrient transfer with rainfall in the canopies of a broad-leaved deciduous forest in Okuchichibu, Journal of Japanese Forest Society, 94, p74-83, (in Japanese).
[https://doi.org/10.4005/jjfs.94.74]

- Kamataki, H., Akiyama, K., Watanabe, T., Ishii, K., Tsukada, Y., Kazama, H., Yoshino, N., (1995), Estimating contribution ratio of sources to suspended particulate matter using chemical mass balance method for regional difference in Tokyo, Annual Report of Tokyo Metropolitan Research Institute, p18-26, (in Japanese).
- Kamataki, H., Komeiji, T., Yamada, M., (2000), Characterization of ion components in atmospheric aerosol in Tokyo district, Nippon Kagaku Kaishi, 12, p891-900, (in Japanese).
-
Kulshrestha, U.C., Sarkar, A.K., Srivastava, S.S., Parashar, D.C., (1995), Wet-only and bulk deposition studies at New Delhi (India), Water, Air, & Soil Pollution, 85, p2137-2142.
[https://doi.org/10.1007/bf01186150]

-
Lai, S., Zou, S., Cao, J., Lee, S., Ho, K., (2007), Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, South China, Journal of Environmental Sciences, 19, p939-947.
[https://doi.org/10.1016/s1001-0742(07)60155-7]

-
Lee, D.S., Longhurst, J.W., (1992), A comparison between wet and bulk deposition at an urban site in the U.K, Water, Air, & Soil Pollution, 64, p635-648.
[https://doi.org/10.1007/bf00483372]

-
Likens, G.E., Driscoll, C.T., Buso, D.C., Siccama, T.G., Johnson, C.E., Lovett, G.M., Ryan, D.F., Fahey, T., Reiners, W.A., (1994), The biogeochemistry of potassium at Hubbard Brook, Biogeochemistry, 25, p61-125.
[https://doi.org/10.1007/bf00000881]

-
Lindberg, S.E., Lovett, G.M., Richter, D.D., Johnson, D.W., (1986), Atmospheric deposition and canopy interactions of major ions in a forest, Science, 231, p141-145.
[https://doi.org/10.1126/science.231.4734.141]

-
Lovett, G.M., Lindberg, S.E., (1984), Dry deposition and canopy exchange in a mixed oak forest as determined by analysis of throughfall, Journal of Applied Ecology, 21, p1013-1027.
[https://doi.org/10.2307/2405064]

- Ministry of Land, Infrastructure, Transport and Tourism, Japan, (2005), Road traffic census 2005, http://www.mlit.go.jp/road/census/h17/ Accessed on 2012 December 17.
-
Mosello, R., Marchetto, A., Tartari, G.A., (1988), Bulk and wet atmospheric deposition chemistry at Pallanza (N. Italy), Water, Air, & Soil Pollution, 42, p137-151.
[https://doi.org/10.1007/bf00282397]

- R Development Core Team, (2009), R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/.
-
Richter, D.D., Lindberg, S.E., (1988), Wet deposition estimates from long-term bulk and event wet-only samples of incident precipitation and throughfall, Journal of Environmental Quality, 17, p619-622.
[https://doi.org/10.2134/jeq1988.00472425001700040017x]

-
Sakugawa, H., Kaplan, I.R., Shepard, L.S., (1993), Measurements of H2 O2, aldehydes and organic acids in Los Angeles rainwater: Their sources and deposition rates, Atmospheric Environment, 27B, p203-219.
[https://doi.org/10.1016/0957-1272(93)90006-r]

- Sakurai, T., Kiyono, T., Nakae, S., Fujita, S., (2002), Analysis of ammonia behavior in the Kanto region, Journal of Japanese Society for Atmospheric Environment, 37, p155-165, (in Japanese).
- Seto City, (2013), Statistical report in Seto City, http://www.city.seto.aichi.jp/docs/2013042200015/ Accessed on 2014 May 27.
-
Staelens, J., De Schrijver, A., Van Avermaet, P., Genouw, G., Verhoest, N., (2005), A comparison of bulk and wet-only deposition at two adjacent sites in Melle (Belgium), Atmospheric Environment, 39, p7-15.
[https://doi.org/10.1016/j.atmosenv.2004.09.055]

-
Stedman, J.R., Heyes, C.J., Irwin, J.G., (1990), A comparison of bulk and wet-only precipitation collectors at rural sites in the United Kingdom, Water, Air, & Soil Pollution, 52, p377-395.
[https://doi.org/10.1007/bf00229445]

-
Swank, W.T., (1984), Atmospheric contributions to forest nutrient cycling, Water Resources Bulletin, 20, p313-321.
[https://doi.org/10.1111/j.1752-1688.1984.tb04710.x]

-
Tanner, P.A., (1999), Analysis of Hong Kong daily bulk and wet deposition data from 1994 to 1995, Atmospheric Environment, 33, p1757-1766.
[https://doi.org/10.1016/s1352-2310(98)00340-9]

- Thimonier, A., (1998), Measurement of atmospheric deposition under forest canopies: Some recommendations for equipment and sampling design, Environmental Monitoring and Assessment, 52, p353-387.
- Yokota, H., Akiyama, K., Miyoshi, T., Ueno, H., Ishii, K., Higuchi, Y., Ito, Y., (2010), Study of estimating of PM2.5 source contribution using Chemical Mass Balance Method, Annual Report of Tokyo Metropolitan Research Institute, p148-149, (in Japanese).