Methane Oxidation in Landfill Cover Soils: A Review
Abstract
Migration of methane (CH4) gas from landfills to the surrounding environment negatively affects both humankind and the environment. It is therefore essential to develop management techniques to reduce CH4 emissions from landfills to minimize global warming and to reduce the human risks associated with CH4 gas migration. Oxidation of CH4 in landfill cover soil is the most important strategy for CH4 emissions mitigation. CH4 oxidation occurs naturally in landfill cover soils due to the abundance of methanotrophic bacteria. However, the activities of these bacteria are influenced by several controlling factors. This study attempts to review the important issues associated with the CH4 oxidation process in landfill cover soils. The CH4 oxidation process is highly sensitive to environmental factors and cover soil properties. The comparison of various biotic system techniques indicated that each technique has unique advantages and disadvantages, and the choice of the best technique for a specific application depends on economic constraints, treatment efficiency and landfill operations.
Keywords:
Methane emissions, Methane oxidation, Mitigation, Methanotrophic bacteria, Cover soils1. INTRODUCTION
Methane (CH4) gas is one of the most important greenhouse gases (GHGs). As a result of human activities, CH4 emission concentrations in the atmosphere have increased from 715 ppb during the pre-industrial age to 1,732 ppb in the early 1990s and 1,774 ppb in 2005 (IPCC, 2007). Although the CH4 concentration in the atmosphere is much lower than that of carbon dioxide (CO2), its global warming potential is 25 times greater (IPCC, 2007). A study by Henckel et al. (2001) showed that the global CH4 concentration is approximately 1.8 ppmv, which represents a doubling during the last 200 years.
Landfills rank as the third major anthropogenic source of CH4 emissions after rice paddies and ruminant manure (Qingxian et al., 2007; Ritzkowski et al., 2007). A total of 40-60 metric tons of CH4 are emitted from landfills worldwide, accounting for approximately 11-12% of the global anthropogenic CH4 emissions (Ritzkowski et al., 2007). CH4 gas migration from landfills to the surrounding environment negatively affects both humankind and the environment. Gas explosion disasters due to landfill gas (LFG) migration resulting from variations in atmospheric pressure were reported in the village of Loscoe in England in 1986 and at Skellingsted Landfill in Denmark (Christophersen et al., 2001).
Mitigation of landfill CH4 emissions has been conducted using two approaches. The first approach uses gas collection systems for recovering or burning LFG, while the second approach seeks to reduce the emissions by various means, including waste recycling, composting and incineration. The first approach is more prevalent because it is cost-effective for large sanitary landfills. However, it is considered to be too costly and infeasible for older and smaller landfills whose CH4 emission rates are much lower. Although major sanitary landfills utilizes gas collection systems, small quantities of LFG still escape into the atmosphere or migrate into the surrounding soil through the topmost layer of cover soil. Some researchers have found that conventional gas recovery systems only capture 50 to 90% of the CH4 generated in landfills (Augenstein and Pacey, 1996). Therefore, the development and application of techniques for effectively reducing CH4 emissions from landfills are required to minimize both the future global warming potential and the human risks associated with CH4 gas emissions.
Microbial CH4 oxidation in landfill cover soil may provide a means of controlling CH4 emissions. Several studies have shown that the CH4 oxidation process in landfill cover is an efficient method of CH4 emission mitigation (Abushammala et al., 2013a; Huber-Humer et al., 2008; Stern et al., 2007; Huber-Humer, 2004; Hilger and Humer, 2003; Humer and Lechner, 1999). This process takes place in many natural systems and soils without human interference, due to the abundance of several groups of bacteria requiring oxygen (O2) for the oxidation process. This process may be exploited to reduce CH4 emissions at landfill sites where gas recovery systems are nonexistent or alongside existing gas collection systems to complement emissions control. A value of 0 to 10% of CH4 oxidation has been recommended by the Intergovernmental Panel on Climate Change (IPCC) guidelines for national GHG inventories. However, laboratory and field studies indicates that the CH4 oxidation capacity is between 0 and 100% (Jugnia et al., 2008). Conversely, Bogner et al. (1995) stated that landfill cover soil under certain conditions can be a sink for atmospheric CH4. Currently, there is insufficient information available regarding CH4 oxidation capacity due to the lack of a standard method to determine the oxidation rate.
This study discusses the CH4 oxidation process, which mitigates CH4 emissions associated with LFG production. First, the mechanisms of CH4 oxidation by methanotrophic bacteria in landfill cover soils are identified. Second, the key factors that control the CH4 oxidation process in landfill cover soils are discussed. Finally, current techniques for mitigating CH4 emissions using biotic systems are compared to investigate their key features and examine how they can be incorporated into the future design of landfill soil covers.
2. METHANE OXIDATION BACTERIA
The CH4 oxidation process in landfill cover soils is facilitated by a group of methanotrophic bacteria that live in landfill cover soil (Huber-Humer, 2004). For simplicity, previous studies have reported that the CH4 oxidation process in landfill cover soils is accomplished by methanotrophic bacteria (Abushammala et al., 2012; Huber-Humer et al., 2008; Albanna et al., 2007; Stern et al., 2007; Kettunen et al., 2006). Methanotrophic bacteria (Fig. 1) are a group of obligate aerobes that have the ability to oxidize CH4 under natural conditions to produce CO2, water (H2O), and microbial biomass (Eq. 1). Other organic compounds in LFG, such as aromatic and halogenated hydrocarbons, can be partially or fully degraded by methanotrophic bacteria that have the ability to co-metabolize substrates other than CH4 (CLEAR, 2009; Scheutz and Bogner, 2003).
| (1) |
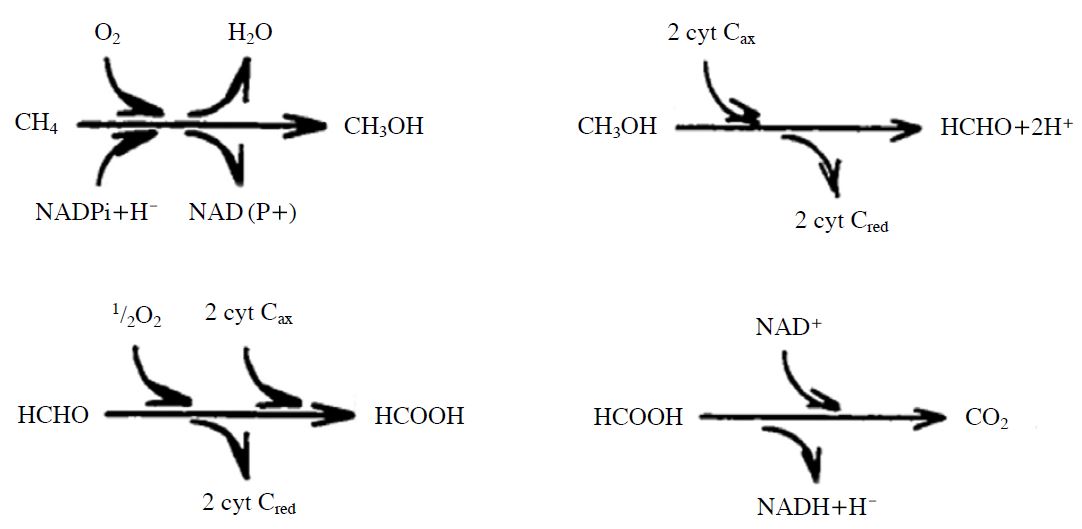
There are several complex enzymatic pathways for CH4 oxidation. Methanotrophs are divided into three types: type I methanotrophs follow a ribulose monophosphate (Ru MP) pathway, type II methanotrophs follow a serine pathway, and type X methanotrophs follow both pathways (Bogner, 1996). These classifications are based on their carbon assimilation pathways, intracytoplasmic membrane arrangements, cell morphology and the specific protein content of their DNA. In general, all three types of methanotrophs possess the CH4 monooxygenase (MMO) enzyme, which assists them in oxidizing CH4 for energy yield (Fig. 2) (Bogner, 1996).
MMO can be found in two forms: particulate CH4 monooxygenase (pMMO) and soluble CH4 monooxygenase (sMMO). Most methanotrophic bacteria are known to express themselves as pMMO, while a few of them express themselves as sMMO, and some have the ability to express themselves in both forms (Lee, 2008). However, methanotrophic bacteria have broad differences with respect to their responses to different CH4 concentrations (Reay and Nedwell, 2004), and they can be classified accordingly as high-affinity or low-affinity methanotrophic bacteria. High-affinity methanotrophic bacteria are characterized by low CH4 oxidation capacity, which enables them to begin oxidation at low CH4 concentrations (0.8-280 nmol L-1) (Huber-Humer et al., 2008). High-affinity methanotrophic bacteria exist in soils temporarily exposed to CH4 concentration. Low-affinity methanotrophic bacteria exhibit a high oxidation capacity, with CH4 levels in the range of 0.8-66 μmol L-1 (Huber-Humer et al., 2008). Low-affinity methanotrophic bacteria are more prevalent in landfill cover soils than are the high-affinity variant (Kightley et al., 1995).
Methanotrophic bacteria can use substrates other than CH4 under certain conditions, resulting in a reduction in the CH4 oxidation rate and oxidation of ammonia (NH4+) to nitrite and nitrous oxide, due to the nonspecific nature of MMO (Knowles, 2005). Bogner (1996) documented inhibitions of methanotrophic activity due to nitrogen cycle processes that occur when hydroxylamine is produced by the oxidation of NH4+ by MMO, which inhibits MMO enzyme activity, when nitrite inhibits other enzyme activity necessary for CH4 oxidation, and finally, when methanol is present in addition to NH4+.
3. FACTORS AFFECTING METHANE OXIDATION
The CH4 oxidation capacity of landfill cover soils varies within and among landfills due to many factors that affect the oxidation process, such as seasonal variations (Abushammala et al., 2013b; Einola et al., 2007; Maurice and Lagerkvist, 2003; Börjesson et al., 2001), physical and chemical heterogeneities of landfill cover soils (Tecle et al., 2008; Albanna et al., 2007; Visvanathan et al., 1999), and the CH4 concentrations in landfills (Boeckx et al., 1996). According to Mosier et al. (2004), the major factors controlling CH4 oxidation are potential biological demand and diffusion. The biological demand is regulated by both the physical and chemical environments, while the CH4 diffusion rate is regulated by physical factors only. The reported values of landfill cover soils’ CH4 oxidizing efficiency vary widely in the literature. Albanna et al. (2007) reported that increasing the soil layer thickness from 15 to 20 cm increased the CH4 oxidation values from 29% to 35% for a soil with 15% moisture content without nutrient addition, from 34% to 38% for a soil with a 30% moisture content without nutrient addition, and from 75% to 81% for a soil with a 30% moisture content with nutrient addition. However, in investigating the effect of bio-cover on CH4 oxidation at the Leon landfill in Florida, Stern et al. (2007) found that the efficiency of CH4 oxidation can reach 64% with bio-cover utilization, while only 30% efficiency was reported for the control cell. Abichou et al. (2009) reported that at the same landfill, an average of 79% of CH4 was oxidized in the bio-cover system and 29% was oxidized in the control cell. These wide variations can be attributed to the previously mentioned factors.
The major controlling environmental factors governing the CH4 oxidation process in landfill cover soils, such as soil texture, organic content, moisture content, temperature, pH, nutrients, and O2 and CH4 concentrations (Wilshusen et al., 2004a; Börjesson et al., 2001; Boeckx et al., 1996) are briefly discussed in this section. Applying knowledge about these controlling factors can optimize the process of mitigating CH4 emissions from landfills.
3. 1 Soil Texture
Soil texture affects LFG transport and atmospheric O2 penetration. It therefore controls both CH4 emission and oxidation rates. The CH4 oxidation capacity in soils of various textures was investigated by Kightley et al. (1995), and it was found that higher oxidation efficiency occurs in coarse sand (61%) than in fine sand or clay (40-41%). Boeckx et al. (1997) concluded that coarse soils have higher oxidizing capacities than fine soils. Gebert and Grongroft (2009) recommended the use of coarse-textured soils with more than 17% air-filled pores by volume, such as sands, loamy sands, sandy loams and some of the coarsely textured loams, for use as CH4 oxidizing bio-cover.
3. 2 Soil Organic Content
The CH4 oxidation rate increases with increasing soil organic content (Humer and Lechner, 2001; Christophersen et al., 2000; Visvanathan et al., 1999). Through soil incubation tests, Christophersen et al. (2000) found that soils containing more organic matter more effectively mitigate CH4 emissions through oxidation. They also found a relationship between the optimal soil moisture content and the organic matter content. The water content provides optimal oxidation increases with increasing organic matter content. Visvanathan et al. (1999) found that higher soil organic contents resulted in higher CH4 oxidation rates in column assays. High-organic-content materials, such as compost, are widely used in landfill cover systems to enrich their CH4 oxidation capacity (Abichou et al., 2009; Huber-Humer et al., 2008; Gebert and Grongroft, 2006a; Wilshusen et al., 2004a; Streese and Stegmann, 2003). Materials with high organic contents, high levels of nutrients, and high porosity have been proven to have high CH4 oxidation capacities, which in some cases, tends to oxidize atmospheric CH4. However, De Visscher et al. (2001) found that adding compost materials enhanced CH4 oxidation, after a brief period of inhibition.
3. 3 Moisture Content
There are several sources of water in landfill soil cover, including surface water infiltration, precipitation, water from manmade sources (leachate recirculation) and the decomposition reaction within the soil cover. As reported previously, a high moisture content in landfill soil cover reduces the available pore space for gaseous transport and diffusion. A high moisture content also reduces O2 penetration into the soil cover, which is the main reactor for the CH4 oxidation process. A low soil moisture content reduces the biological activity in soil cover and results in a reduction in CH4 oxidation capacity (Tecle et al., 2008). The combination of soil drying due to low moisture content and the heat generated by CH4 oxidation are likely to reduce the pore water content of soil, which may facilitate LFG transport through the shallow soil cover and reduce the oxidation capacity, due to the inhibition of microbiological activities that require a certain amount of water (Maurice and Lagerkvist, 2003). The desirable moisture content for high CH4 oxidation activity is in the range of 11-25% by volume (Tecle et al., 2008). Boeckx et al. (1996) studied the effect of the soil moisture content on the CH4 oxidation capacity of a landfill soil cover 30 cm thick. In his laboratory test, the moisture content of the soil was tested at 5, 10, 15, 20, 25 and 30% by weight, and the optimum moisture content was found to be between 15.6 and 18.8% by weight. Visvanathan et al. (1999) reported ideal moisture contents of 15% and 15 to 20% for maximum CH4 oxidation in column and batch experiments, respectively. They stated that a negligible amount of CH4 oxidation might occur at a 6% moisture content and that zero oxidation would occur at a 1.5% moisture content. Lee et al. (2009) found that the highest CH4 oxidation rates occurred at a moisture content of 5% in a sandy landfill soil cover, with CH4 oxidation rates decreasing as the moisture content increased.
Four sandy soils from two landfills in Denmark were investigated in batch experiments by Christophersen et al. (2000) to determine the effects of soil moisture on CH4 oxidation. The results showed that the optimum moisture content range from 11 to 32% in all samples. It was also found that both moisture content and CH4 oxidation increased as the organic matter content increased. More recently, work has been conducted by Park et al. (2002) to test the effect of the moisture content of loamy sandy soil on CH4 oxidation capacity. They found that 13% by weight was the optimum moisture content for CH4 oxidation in this soil. Another study conducted by Park et al. (2005) concluded that moisture content is the most important factor controlling the CH4 oxidation rate is a sandy soil landfill cover. Mor et al. (2006) found that the effect of the soil moisture content on CH4 oxidation in various types of compost was time-dependent and that the optimum moisture content ranges between 45 and 110% (dry weight basis).
3. 4 Temperature
CH4 oxidation in landfill soil cover is a biological process, and soil temperature is an important factor affecting this process (Streese and Stegmann, 2003). The methanotrophic community structure changes due to temperature variations, rather the quantity of type II methanotrophs decreasing with increasing temperature and precipitation (Horz et al., 2005). Several studies have reported on the optimum temperature for CH4 oxidation in soil cover. Castro et al. (1995) found that soil temperature is an important factor in CH4 oxidation at temperatures between -5°C and 10°C but has no effect on CH4 oxidation at temperatures between 10°C and 20°C. Visvanathan et al. (1999) documented inhibition of CH4 oxidation at temperatures higher than the optimum temperature, which they found in laboratory experiments to be in the range of 30 to 36°C. De Visscher et al. (2001) confirmed these results in reporting that 35°C was found to be the optimum temperature for CH4 oxidation activity in a sandy loamy soil from a landfill in Belgium. They also concluded that soil temperatures in excess of 30°C for long periods can lead to a reduction in CH4 oxidation activity. Scheutz and Kjeldsen (2004) reported that CH4 oxidation increased exponentially (with R2>0.91) with increases in soil temperature from 2 to 25°C. The maximum CH4 oxidation rate occurred at 30°C, and the oxidation rate started to decline at 40°C. The effect of temperature on CH4 oxidation in various types of compost was studied by Mor et al. (2006), who found that the effect of temperature on CH4 oxidation is time-dependent and that the optimum temperature range is between 15 to 30°C. Borken et al. (2006) found that in forest soils, summer drought may increase CH4 oxidation.
On the other hand, it has been reported that there is an interdependency between the effects of soil temperature and water content on CH4 oxidation. Visvanathan et al. (1999) found that a sufficient moisture content combined with an appropriate temperature (approximately 20°C) could result in higher CH4 oxidation. However, Castaldi and Fierro (2005) found that CH4 oxidation rates were maximized when the water content was very low and the temperature was high. Einola et al. (2007) have reported an interdependency between soil temperature and water content, the most important factors controlling CH4 oxidation capacity, and their effects on CH4 oxidation.
3. 5 pH
Variation in the pH value of a landfill soil cover affects CH4 oxidation activities (Hutsch et al., 1994). According to Whittenbury et al. (1970), all types of methanotrophic bacteria can grow in pH values ranging from 5.8 to 7.4, with the optimum pH value being in the range of 6.6 to 6.8. However, Saari et al. (2004), found the optimum pH for CH4 oxidation to vary from 4 to 7.5 in tests of CH4 oxidation capacity in different type of soils with pH values ranging from 3 to 7.5. They also found that for some soils, the optimum pH for CH4 oxidation is greater than the natural pH. The optimal pH value for CH4 oxidation in soil samples collected from the Skellingsted Landfill in Denmark was found by Scheutz and Kjeldsen (2004) to be 6.9.
Methanotrophic bacteria are sensitive to the acidification of surrounding soils. Mer and Roger (2001) observed that the oxidation rate of non-fertilized permanent grassland at the Rothamsted experimental station in England decreased from -67 to -35 nL CH4.L-1.h-1 (nL=nanoliter) when the pH of the cover soil at the site decreased from 6.3 to 5.6. Others have reported that the CH4 oxidation decreases to zero at pH values between 5.6 and 5.1 (Huetsch et al., 1994). According to Hanson and Hanson (1996), methanotrophic bacteria cannot grow at pH values below 5. Numerous attempts to isolate or obtain enrichments for methanotrophic bacteria that would grow at pH values below 5.5 from acidic peat samples have failed.
3. 6 Nutrients
Aside from the carbon substrate from CH4 oxidation, bacteria in landfill soil cover require other nutrients for their cellular metabolism. The addition of nutrients to a soil cover system results in activation of methanotrophic bacteria, thus enhancing the CH4 oxidation rate and oxidation efficiency (Lee et al., 2009; Albanna et al., 2007; Börjesson et al., 1998).
Albanna et al. (2007) found that soil moisture and the addition of nutrients have a combined effect on CH4 oxidation in soil cover, and they reported that adding nutrients to incubated soil with a 32% average moisture content doubles the oxidation efficiency. However, adding nutrients to a soil with a low moisture content (15%) was found to have a negative effect on the oxidation efficiency. Lee et al. (2009) found that the CH4 oxidation capacity of sandy soil cover increased by approximately 60% with the addition of 100 mg-N NH4+ per kg of soil.
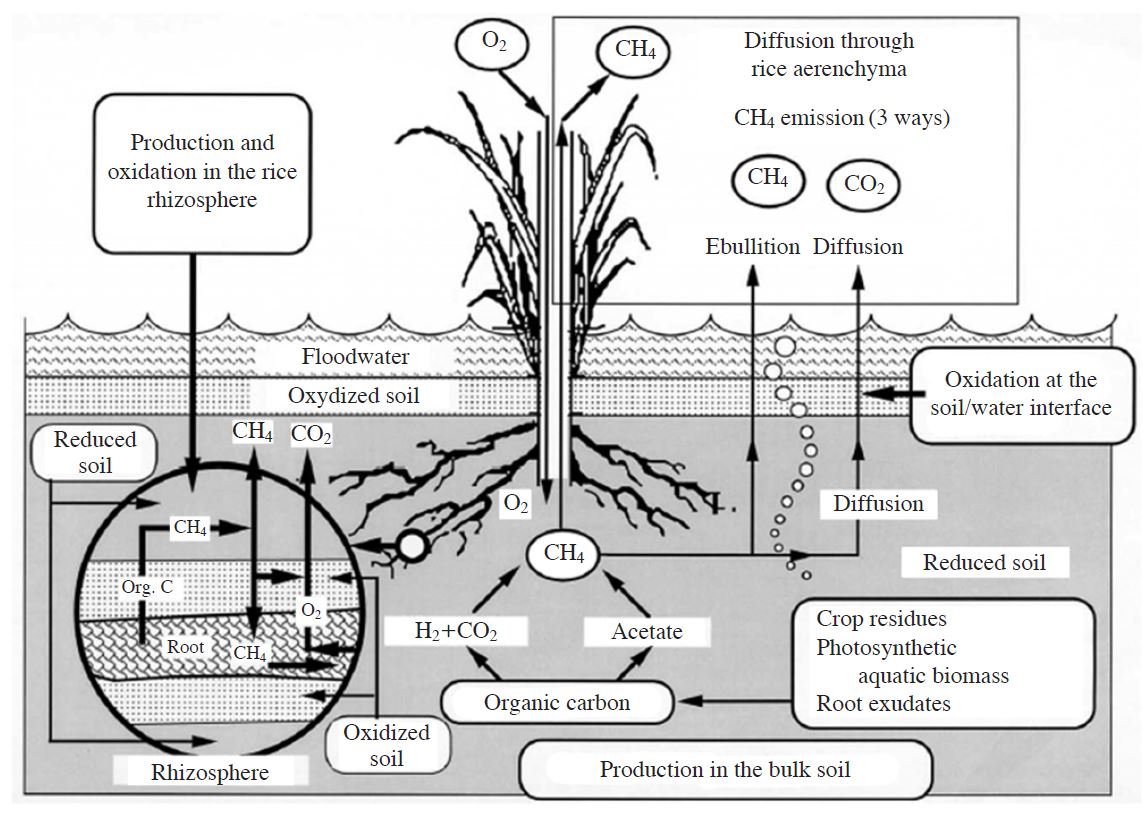
Vegetation might affect on the growth and activity of methanotrophic bacteria in a variety of ways (Wang et al., 2008). Vegetation roots assist the process of transporting O2 from the atmosphere into deeper soil layers (Fig. 3) (Tanthachoon et al., 2007). Furthermore, exudates that are supportive nutrients for methanotrophic bacteria are released to the root zone, which enhances CH4 oxidation (Tanthachoon et al., 2007). Therefore, vegetation on the surfaces of landfill covers encourages methanotrophic activities throughout the soil depth profile. However, vegetation might compete with microorganisms for nutrients and water, which might result in an overall decrease in CH4 oxidation (Hilger and Humer, 2003). Bohn and Jager (2009) found that the CH4 oxidation rate can be enhanced by at least 50% by vegetation growth on landfill cover soils.
In engineered biological treatment systems, nitrogen and phosphorous is added in the form of NH4+ and orthophosphate. Adding NH4+ reduces the CH4 oxidation capacity due to NH4+ inhibiting the activities of methanotrophic bacteria (Reay and Nedwell, 2004; Wang and Ineson, 2003; Hanson and Hanson, 1996). However, as discussed previously, the oxidation of NH4+ produces nitrite, which has an inhibitory effect on the MMO enzyme. Bosse et al. (1993) found that the CH4 oxidation rate decreases at NH4+ concentrations ›4 mM (mM=millimolar) and is completely inhibited at NH4+ concentrations >20 mM. Keller et al. (2006) reported that nutrients (nitrogen and phosphorus) are important in the control of peat land microbial carbon cycling and that the roles of these nutrients differ with short- and long-term incubation.
3. 7 Oxygen Concentration
Oxygen is one of the main reactors and limiting factors controlling the CH4 oxidation process in landfill cover soils (Berger et al., 2005). The O2 concentration varies with the depth of soil cover and is influenced by many variables, including gas characteristics, meteorological conditions, the microbial CH4 oxidation rate, the soil texture and the cover thickness. Soil porosity controls the depth of O2 penetration into soil (Humer and Lechner, 1999). The overlapping of the gradients of the CH4 and O2 concentrations in a soil profile occurs at the point of maximum CH4 oxidation, and the depth at which this overlapping occurs is the optimum depth for maximum CH4 oxidation. Several researchers have found different maximum CH4 oxidation zones. Visvanathan et al. (1999) found that maximum oxidation occurs at depths of 15 to 40 cm, while Börjesson and Svensson (1997) found that 50 to 60 cm is the optimum depth for maximum CH4 oxidation. A study conducted by Jones and Nedwell (2006) stated that the maximum CH4 oxidation occurred at depths from 10 to 30 cm, while Jugnia et al. (2008) stated that 0-10 cm is the optimal depth for CH4 oxidation activity. William and Zobell (1949) reported that O2 concentrations between 10 to 40% produce the highest range of CH4 oxidation rates (Table 1), with an increase or decrease in O2 concentration outside this range decreasing the CH4 oxidation rate.
3. 8 Methane Concentration
The influence of the CH4 concentration on the CH4 oxidation capacity can be described using the Michaelis-Menten equation (Eq. 2):
| (2) |
where V is the actual CH4 oxidation rate (m3 m-3 s-1), Vmax is the maximum CH4 oxidation rate (m3 m-3 s-1), KM is the Michaelis constant for CH4 (%) and C is the CH4 concentration (%). Many researchers have reported the effect of the CH4 concentration on the CH4 oxidation capacity (Pawlowska and Stepniewski, 2006; Visvanathan et al., 1999; Bogner et al., 1997). Pawlowska and Stepniewski (2006) documented a significant influence of CH4 concentration on the CH4 oxidation capacity through a bio-filter model assay. They found that an eightfold increase in CH4 concentration caused the CH4 oxidation capacity to increase by a factor of 1.1 to 2.5. Visvanathan et al. (1999) studied, in both column and batch assays, the effects of different environmental factors, such as soil temperature, moisture content and CH4 concentration on the CH4 oxidation capacity of landfill cover soils. They found that the CH4 supply rate in column assays and the CH4 concentration in the headspace of batch assays conflicts were different for low and high CH4 oxidation capacities, due to the effects of both soil moisture content and temperature on the CH4 oxidation capacity.
4. BIOTIC SYSTEMS FOR CH4 OXIDATION
LFG treatment using a variety of types of biotic systems, including bio-washers (Figueroa, 1996), bio-membranes (Figueroa, 1996), bio-filters (Huber-Humer et al., 2008; Gebert and Grongroft, 2006a; Wilshusen et al., 2004b; Streese and Stegmann, 2003; Figueroa, 1996), bio-windows (Huber-Humer et al., 2008), bio-covers (Shangari and Agamuthu, 2012; Huber-Humer et al., 2008) and bio-tarps (Huber-Humer et al., 2008), has been discussed in the literature. The first two types of systems (bio-washers and bio-membranes) for landfill emissions treatment are not discussed in this section because of their limited use. Biotic systems such as bio-filters, bio-windows, bio-covers and bio-tarps are discussed in more detail as they are the most widely used types of systems.
Biotic systems are economical options for controlling low levels of CH4 emissions from landfills. Biotic systems can be used in many applications in landfills, in addition to gas collection systems for trapping CH4 emissions at old landfills, at small landfill sites at which gas collection systems are not economical options and during landfill site postclosure and aftercare processes.
Biotic systems used for CH4 emissions mitigation are described in the following sections in terms of their key features and their incorporation into the design of future landfill cover soils.
4. 1 Bio-Filter
Bio-filters were first used for contaminated gas treatment in the USA in 1966 to deodorize sewage sludge digestion gas. Recently, the application of bio-filters has expanded to CH4 oxidation of LFG in addition to odor elimination. The first application of bio-filters for LFG treatment on a laboratory scale to investigate deodorization and the degradation of both H2S and CH4 was in 1979. Aerobic degradation of CH4 in LFG using bio-filters was first investigated in 1986 (Figueroa, 1996).
Several laboratory and field experiments have been conducted to investigate bio-filter designs, media and gas flow. The filters are operated either in open or fully contained beds. A bio-filter consists primarily of a filter material that influences the performance of purification by its physical, chemical and biological properties (Figueroa, 1996). This filter material is considered to be the most important part of a bio-filter system because it supports bacteria cultures and is capable of sorption of contaminated gas. Bio-filter materials are primarily of biological origin, such as peat, compost from bio-waste, heather, shredded bark and sawdust (Huber-Humer et al., 2008; Figueroa, 1996). Bio-filters have high water storage capacity and sufficient nutrients to facilitate biological processes. Admixtures such as expanded clay, polystyrene, lava and active carbon can be added to improve the structure of the filter material and increase its purification efficiency. LFG passively vented through the pressure gradient between the landfill and the atmosphere (Gebert and Grongroft, 2006a) can be directed through the filter in either of two modes (Huber-Humer et al., 2008): upflow or downflow (Fig. 4).
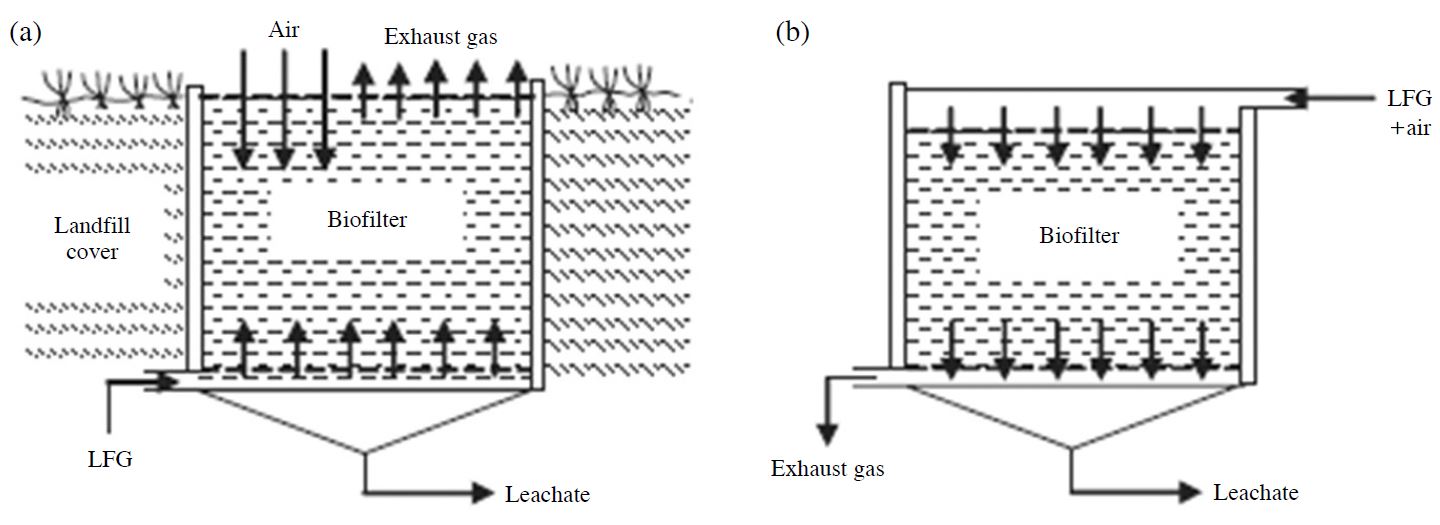
Various integrated design of bio-filters and landfill soil cover: (a) upflow mode and (b) downflow mode.
The CH4 degradation process in a bio-filter is highly dependent on the retention time of LFG inside the filter (i.e., gas flow). Figueroa (1996) found 50 g CH4 m-3 h-1 removal at a surface load of 5m h-1 and complete CH4 removal at a surface load of 0.5 m h-1. However, several environmental conditions affect the filter efficiency (Figueroa, 1996), such as water content, temperature, pore volume or residence time and filter resistance. Good control of these environmental factors results in high filter efficiency and a positive effect on the functions of microorganisms.
CH4 oxidation rates in the range of 20-60 g m-3 h-1 have been observed in a variety of laboratory column studies of bio-filters (Wilshusen et al., 2004a; Streese and Stegmann, 2003; Park et al., 2002), including studies up to one year in length. Wilshusen et al. (2004a) studied several types of compost filter material using column experiments conducted over periods up to 220 days on a laboratory scale to compare their CH4 oxidation potential. They observed that a maximum of 400 g CH4 m-2 day-1 CH4 oxidized over a period of 100 days, followed by a decrease in rate to approximately 100 g CH4 m-2 day-1 over the next 120 days. Various bio-filter materials for LFG treatment were tested by Streese and Stegmann (2003). They found that a mixture of compost, peat, and wood fibers exhibited a stable CH4 oxidation rate of approximately 20 g m-3 h-1 for a CH4 concentration of 3% by volume over a period of one year. On the other hand, finegrained compost used as a bio-filter material was reported by the same authors to result in a CH4 removal rate of up to 63 g m-3 h-1 in the first three months of the experiment for a CH4 concentration of 2.5% by volume. Later, in the fifth month of the experiment, the decrease in the CH4 oxidation rate was monitored. Both Wilshusen et al. (2004a) and Streese and Stegmann (2003) attributed the reduction in the CH4 oxidation rate after reaching its maximum level to extracellular polymeric substances (EPS) formed by methanotrophic microorganisms.
EPS formation is a serious problem with bio-filters (Huber-Humer et al., 2008; Gebert and Grongroft, 2006b; Wilshusen et al., 2004a; Streese and Stegmann, 2003). These substances can block the pore space of the filter material and delay the substrate supplementation to the microorganisms inside the filter material, resulting in the deceleration of methanotrophic activity. EPS formation occurs primarily as a consequence of prolonged use of an active gas feed system(Wilshusen et al., 2004a; Streese and Stegmann, 2003). Passive bio-filters tends to receive gas in an intermittent manner. However, by controlling the inlet flux rate to a landfill bio-filter, it may be possible to mitigate or prevent EPS formation (Huber-Humer et al., 2008). Nonetheless, usage of additional gas distribution layers in bio-filter material optimizes mass transfer of gas components, thus reducing EPS formation (Streese and Stegmann, 2003). Hilger et al. (2009) reported that a nutrient imbalance could promote EPS formation in a bio-filter system.
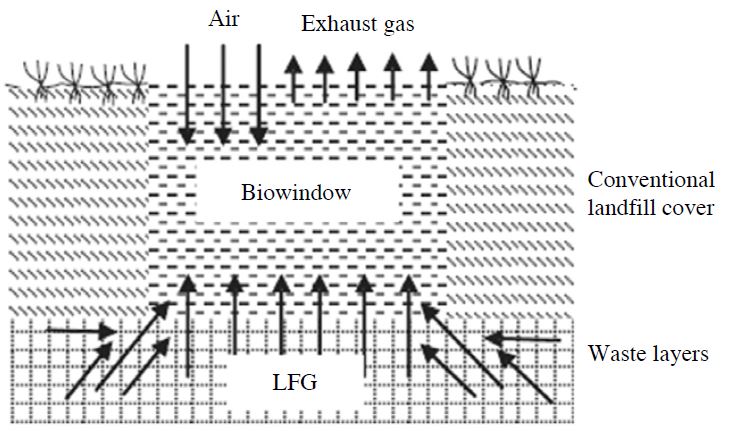
4. 2 Bio-Window
A bio-window is a system for mitigating landfill CH4 emissions to the atmosphere. Composted materials with adopted environmental conditions are usually used as bio-window media to attain maximum CH4 oxidation efficiency through enhanced microbial activity by CH4 oxidation bacteria. The bio-window (Fig. 5) is integrated with the landfill soil cover in small regions of a landfill where high CH4 emissions are observed. Measurements of the spatial variability of CH4 emissions from landfill cover soils using the flux chamber technique and geo-statistical analysis are used to identify CH4 emission hot spots within a landfill. Incorporation of a bio-window system into a landfill soil cover in these zones greatly mitigates the CH4 emissions of the entire landfill. This technique is useful when the use of full-expanse compost materials is not economically feasible and when no gas collection system is available to feed a bio-filter system (Huber-Humer et al., 2008). A bio-window receives passively vented LFG from the underlying waste, thereby offering flexible routes for gas movement.
4. 3 Bio-Cover
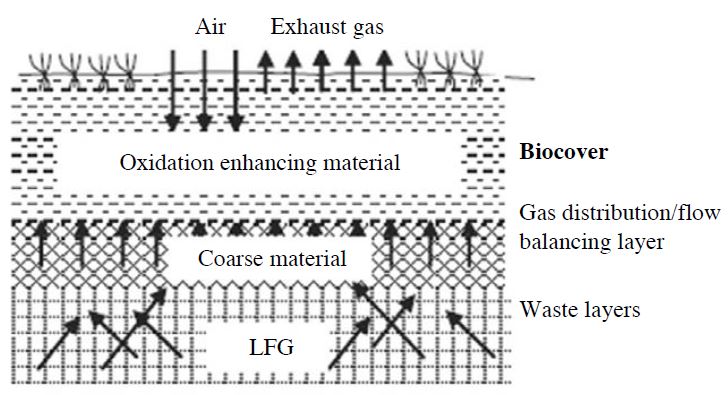
In 2009, Huber-Humer et al. defined a landfill bio-cover as a top cover that optimizes the environmental conditions for methanotrophic bacteria and enhances biotic CH4 consumption. A typical bio-cover system consists of a highly porous gas distribution layer above the waste, often gravel or crushed glass, followed by a compost-amended layer. The thickness of the gas distribution layer usually ranges from 10 to 30 cm (Jugnia et al., 2008; Stern et al., 2007), while the compost layer in the upper part is thicker, up to 100 cm or more, to attain high oxidation capacity. The gas distribution layer above the waste results in uniform LFG fluxes to the bio-cover layer, which permits biological activity to occur in a typical manner (Fig. 6).
Many researchers have attempted to reduce landfill CH4 emissions to the atmosphere using bio-cover systems (Shangari and Agamuthu, 2012; Bogner et al., 2010; Abichou et al., 2009; Huber-Humer, 2009; Jugnia et al., 2008; Stern et al., 2007; Bogner et al., 2005; Huber-Humer, 2004; Hilger and Humer, 2003; Humer and Lechner, 2001; Humer and Lechner, 1999). Their results show high CH4 oxidation capacity in diverse, mature and well-structured compost materials, in both laboratory investigations (Abichou et al., 2009; Stern et al., 2007) and field trials (Bogner et al., 2005; Huber-Humer, 2004; Humer and Lechner, 2001; Humer and Lechner, 1999). Shangari and Agamuthu (2012) found that CH4 oxidation can reach 100% when a bio-cover of brewery spent grain and compost materials is used at a ratio of 7 : 3. Abichou et al. (2009) found that 100% CH4 oxidation capacity can be achieved using compost bio-cover as a landfill cover. Humer and Lechner (2001) reported that the CH4 oxidation capacity of compost landfill cover can reach 100% under optimum conditions of proper design and compost quality. Berger et al. (2005) found that in cover soil consisting of two layers, a mixture of compost plus sand (0.3 m) over a layer of loamy sand (0.9 m), the CH4 oxidation capacity ranged from 98% to 57%. A system consisting of 50 cm of pre-composted yard bio-cover placed over 10-15 cm of crushed glass, utilized as a gas distribution layer, over a 40-100 cm interim cover, was used by Stern et al. (2007) to investigate its landfill CH4 emission reduction and CH4 oxidation capacity. They found that the bio-cover cells reduced CH4 emissions by a factor of 10 and doubled the percentage of CH4 oxidation relative to control cells.
4. 4 Bio-Tarp
There are two types of cover that are used in landfills before final capping. The first type is referred to as a daily cover and the second type is referred to as an intermediate cover. On an operational landfill site, a daily cover is used to cover the in-place waste at the end of each working day. An intermediate soil cover is used after a cell is completed and is awaiting final capping.
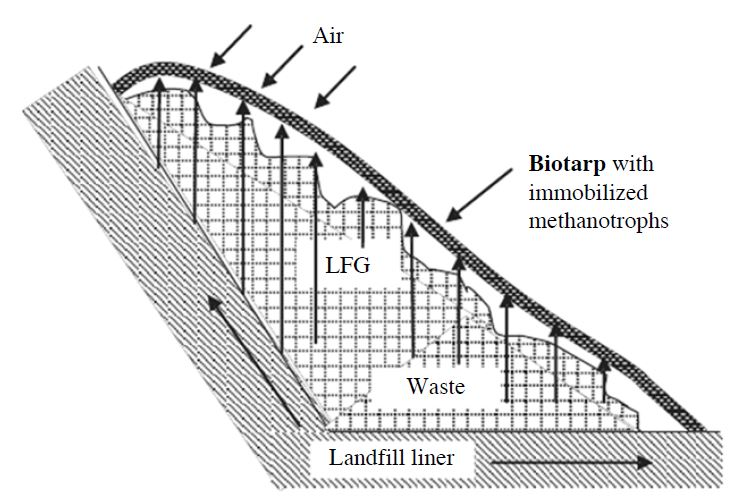
The daily cover functions to prevent interaction between the waste and air, thereby reducing odors. Furthermore, the daily cover is important to prevent windblown litter, minimize the risk of fire within the site, and discourage scavengers and flies. Most landfills use a 15-cm soil layer as a daily cover (Hilger et al., 2009; Huber-Humer et al., 2008). Alternative daily cover (ADC) materials, such as green and brown waste, sewage sludge, water slurries or commercial products such as foams and canvas, can also be used. The use of ADC materials are appropriate at some sites where local soils are unavailable and additional air space is required. Tarps are one type of ADC that maximizes airspace and thereby minimizes the required volume required of any other daily cover. Tarps are placed at the end of the working day and removed the next day to allow for further waste deposition. The filling of an active landfill cell may take a long period of time, during which no CH4 collection occurs. In this case, the use of a bio-tarp (Fig. 7) is a good strategy for mitigating CH4 emissions via methanotrophic bacteria impregnated in its material. Adams et al. (2011) found that the use of multiple layers of water-absorbent geotextiles as bio-tarps removed 16% of CH4, while adding landfill cover soil, compost or shale amendments to the bio-tarp increased the CH4 removal by up to 32%.
Unlike bio-filters, bio-windows and bio-covers, bio-tarps can be removed and re-activated and can serve as a portable emissions reduction strategy. A comparison of the aforementioned biotic systems is provided in Table 2.
5. CONCLUSIONS
This study discusses the CH4 oxidation process, which mitigates CH4 emissions associated with LFG production. Many factors affect the CH4 oxidation capacity of landfill soil cover. The most important factors are environmental factors and the properties of the cover soil. Special consideration must be given to those factors to enhance the CH4 oxidation process and to mitigate landfill CH4 emissions.
Biotic systems are economically feasible options for controlling low levels of CH4 emissions from landfills. Based on the summary table (Table 2) in which the various types of biotic systems are compared, biofilters appear to be appropriate at landfills where LFG collection is in operation because of their high CH4 uptake capacity. Bio-covers offer the advantage of covering an entire landfill while simultaneously providing good water-holding capacity and porosity for vegetation and evapotranspiration. Bio-windows can be used at landfill hotspots. Bio-tarps can be appropriate alternative daily covers for use in mitigating CH4 emissions during landfill operations at times when no CH4 collection occurs. Each type of biotic system has advantages and disadvantages, and the choice of which method to apply depends on economic constraints, treatment efficiency and landfill operations.
Acknowledgments
This work was financed by Universiti Kebangsaan Malaysia under research grant UKM-GUP-ASPL-08-06-208.
References
-
Abichou, T., Mahieu, K., Yuan, L., Chanton, J., Hater, G., (2009), Effects of compost bio-covers on gas flow and methane oxidation in a landfill cover, Waste Management, 29, p1595-601.
[https://doi.org/10.1016/j.wasman.2008.11.007]

- Abushammala, M.F.M., Basri, N.E.A., Basri, H., Kadhum, A.A.H., El-Shafie, A.H., (2012), Empirical gas emission and oxidation measurement at cover soil of dumping site: example from Malaysia, Monitoring and Assessment, 185, p4919-4932.
- Abushammala, M.F.M., Basri, N.E.A., Elfithri, R., (2013a), Assessment of methane emission and oxidation at Air Hitam Landfill site cover soil in wet tropical climate, Environmental Monitoring and Assessment, 185, p9967-9978.
-
Abushammala, M.F.M., Basri, N.E.A., Kadhum, A.A.H., Basri, H., El-Shafie, A.H., Sharifah Mastura, S.A., (2013b), Evaluation of methane generation rate and potential from selected landfills in Malaysia, International Journal of Environmental Science and Technology, in press.
[https://doi.org/10.1007/s13762-013-0197-0]

-
Adams, B.L., Besnard, F., Bogner, J., Hilger, H., (2011), Bio-tarp alternative daily cover prototypes for methane oxidation atop open landfill cells, Waste Management, 31, p1065-1073.
[https://doi.org/10.1016/j.wasman.2011.01.003]

-
Albanna, M., Fernandes, L., Warith, M., (2007), Methane oxidation in landfill cover soil; the combined effects of moisture content, nutrient addition, and cover thickness, Journal of Environmental Engineering Science, 6, p191-200.
[https://doi.org/10.1139/s06-047]

- Augenstein, D., Pacey, J., (1996), Landfill Gas Energy Utilization: Technology Options and Case Studies, United State Environmental Protection Agency, Office of Air and Radiation, p1-4, EPA-600/R-92-116.
-
Berger, J., Fornes, L.V., Ott, C., Jager, J., Wawra, B., Zanke, U., (2005), Methane oxidation in a landfill cover with capillary barrier, Waste Management, 25, p369-373.
[https://doi.org/10.1016/j.wasman.2005.02.005]

-
Boeckx, P., Cleemput, O.V., Villaralvo, I., (1996), Methane emission from a landfill and the methane oxidizing capacity of its covering soil, Soil Biology and Biochemistry, 28, p1397-1405.
[https://doi.org/10.1016/s0038-0717(96)00147-2]

- Boeckx, P., Cleemput, O.V., Villaralvo, I., (1997), Methane oxidation in soils with different textures and land use, Nutrient Cycling in Agroecosystems, 49, p91-95.
-
Bogner, J., Spokas, K., Burton, E., Sweeney, R., Corona, V., (1995), Landfills as atmospheric methane sources and sinks, Chemosphere, 31, p4119-4130.
[https://doi.org/10.1016/0045-6535(95)80012-a]

- Bogner, J., Spokas, K., Chanton, J., Powelson, D., Fleiger, J., Abichou, T., (2005), Modeling landfill methane emissions from bio-covers: a combined theoretical-empirical approach, In: Proceedings Sardinia ’05 - Tenth International Waste Management and Landfill Symposium, CISA, Cagliari, Italy, 3-7 October 2005.
- Bogner, J.E., (1996), Rates of greenhouse gas emission at the Mallard Lake Landfill, Dupage country, Illinois-Major controls and implications for global methane budgets, Doctoral Thesis at the Northern Illinois University, Dekalb, Illinois 1996.
-
Bogner, J.E., Chanton, J.P., Blake, D., Abichou, T., Powelson, D., (2010), Effectiveness of a Florida Landfill Biocover for Reduction of CH4 and NMHC Emissions, Environmental Science and Technology, 44, p1197-1203.
[https://doi.org/10.1021/es901796k]

-
Bogner, J.E., Spokas, K.A., Burton, E.A., (1997), Kinetics of Methane Oxidation in a Landfill Cover Soil: Temporal Variations, a Whole-Landfill Oxidation Experiment, and Modeling of Net CH4 Emissions, Environmental Science and Technology, 31(9), p2504-2514.
[https://doi.org/10.1021/es960909a]

- Bohn, S., Jager, J., (2009), Micronial methane oxidation in landfill top covers-Process study on an MBT landfill, In: Proceedings Sardinia, Twelfth International Waste Management and Landfill Symposium, CISA, Cagliari, Italy, 5-9 October 2009.
-
Börjesson, G., Chanton, J., Svensson, B.H., (2001), Methane oxidation in two Swedish Landfill covers measured with carbon-13 to carbon-12 isotope ratios, Journal of Environmental Quality, 30, p369-376.
[https://doi.org/10.2134/jeq2001.302369x]

- Börjesson, G., Sundh, I., Tunlid, A., Frostegard, A., Svensson, B.H., (1998), Microbial oxidation of CH4 at high partial pressures in an organic landfill cover soil under different moisture regimes, FEMS Microbiology Ecology, 26, p207-217.
-
Börjesson, G., Svensson, B.H., (1997), Seasonal and diurnal methane emissions from a landfill and their regulation by methane oxidation, Waste Management and Research, 15, p33-54.
[https://doi.org/10.1177/0734242x9701500104]

-
Borken, W., Davidson, E.A., Savage, K., Sundquist, E.T., Steudler, P., (2006), Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil, Soil Biology and Biochemistry, 38, p1388-1395.
[https://doi.org/10.1016/j.soilbio.2005.10.011]

-
Bosse, U., Frenzel, P., Conrad, R., (1993), Inhibition of methane oxidation by ammonium in the surface layer of a littoral sediment, FEMS Microbiology Ecology, 13, p123-134.
[https://doi.org/10.1111/j.1574-6941.1993.tb00058.x]

-
Castaldi, S., Fierro, A., (2005), Soil-atmosphere methane exchange in undisturbed and burned mediterranean shrubland of Southern Italy, Ecosystems, 8, p182-190.
[https://doi.org/10.1007/s10021-004-0093-z]

-
Castro, M.S., Steudler, P.A., Melillo, J.M., Aber, J.D., Bowden, R.D., (1995), Factors controlling atmospheric methane consumption by temperate forest soils, Global Biogeochemical Cycles, 9, p1-10.
[https://doi.org/10.1029/94gb02651]

-
Christophersen, M., Kjeldsen, P., Holst, H., Chanton, J., (2001), Lateral gas transport in soil adjacent to an old landfill: factors governing emissions and methane oxidation, Waste Management and Research, 19, p595-612.
[https://doi.org/10.1177/0734242x0101900616]

-
Christophersen, M., Linderod, L., Jensen, P.E., Kjeldsen, P., (2000), Methane oxidation at low temperatures in soil exposed to landfill gas, Journal of Environmental Quality, 29, p1989-1997.
[https://doi.org/10.2134/jeq2000.00472425002900060036x]

- CLEAR, (2009), Consortium for Landfill Emissions Abatement Research, Proposal. International Working Group, http://ch4ox.lmem.us/clear.pdf (17 may 2010).
-
De Visscher, A., Schippers, M., Cleemput, O.V., (2001), Short-term kinetic response of enhanced methane oxidation in landfill cover soils to environmental factors, Biology and Fertility of Soils, 33, p231-237.
[https://doi.org/10.1007/s003740000313]

-
Einola, J.K., Kettunen, R.H., Rintala, J.A., (2007), Responses of methane oxidation to temperature and water content in cover soil of a boreal landfill, Soil Biology and Biochemistry, 39, p1156-1164.
[https://doi.org/10.1016/j.soilbio.2006.12.022]

- Figueroa, R.A., (1996), Landfill gas treatment by biofilters, In Christensen, T.H., Cossu, R., and Stegmann, R., (eds)Landfilling of Waste, Biogas, E and FN Spon, p535-549.
- Gebert, J., Grongroft, A., (2006a), Passive landfill gas emission - Influence of atmospheric pressure and implications for the operation of methane-oxidising biofilters, Waste Management, 26, p245-251.
- Gebert, J., Grongroft, A., (2006b), Performance of a passively vented field-scale biofilter for the microbial oxidation of landfill methane, Waste Management, 26, p399-407.
- Gebert, J., Groengroeft, A., (2009), Role of Soil Gas Diffusivity for the Microbial Oxidation of Methane in Landfill Covers, In: Proceedings Sardinia 2009, Twelfth International Waste Management and Landfill Symposium, CISA, Cagliari, Italy, 5-9 October 2009.
- Hanson, R.S., Hanson, T.E., (1996), Methanotrophic Bacteria, Microbiological Reviews, 60, p439-471.
-
Henckel, T., Jackel, U., Conrad, R., (2001), Vertical distribution of the methanotrophic community after drainage of rice field soil, FEMS Microbiology Ecology, 34, p279-291.
[https://doi.org/10.1016/s0168-6496(00)00105-7]

- Hilger, H., Humer, M., (2003), Biotic landfill cover treatments for mitigating methane emissions, Environmental Monitoring and Assessment, 84, p71-84.
- Hilger, H., Oliver, J., Bogner, J., Jones, D., (2009), Reducing open sell landfill methane emissions with a bioactive alternative daily cover, Final Scientific Report, August 1, 2005-March 31, 2009. Department Of Environment (DOE): 2010, p84, Report No.: DE-FC26-05NT42433.
-
Horz, H.P., Rich, V., Avrahami, S., Bohannan, B.J.M., (2005), Methane-oxidizing bacteria in a California upland grassland soil: Diversity and response to simulated global change, Applied and Environmental Microbiology, 71, p2642-2652.
[https://doi.org/10.1128/aem.71.5.2642-2652.2005]

-
Huber-Humer, M., (2004), International research into landfill gas emissions and mitigation strategies-IWWG working group “CLEAR”, Waste Management, 24, p425-427.
[https://doi.org/10.1016/j.wasman.2004.02.005]

-
Huber-Humer, M., Gebert, J., Hilger, H., (2008), Biotic systems to mitigate landfill methane emissions, Waste Management and Research, 26, p33-46.
[https://doi.org/10.1177/0734242x07087977]

-
Huber-Humer, M., Roder, S., Lechner, P., (2009), Approaches to assess bio-cover performance on landfills, Waste Management, 29, p2092-2104.
[https://doi.org/10.1016/j.wasman.2009.02.001]

-
Humer, M., Lechner, P., (1999), Alternative approach to the elimination of greenhouse gases from old landfills, Waste Management and Research, 17, p443-452.
[https://doi.org/10.1034/j.1399-3070.1999.00064.x]

- Humer, M., Lechner, P., (2001), Design of a landfill cover layer to enhance methane oxidation results of a two year field investigation, In: Proceedings of “SARDINIA 2001 - Eighth International Waste Management and Landfill Symposium”, Leachate and Landfill Gas, vol. II. CISA, Cagliari p541-550.
-
Hütsch, B.W., Webster, C.P., Powlson, D.S., (1994), Methane oxidation in soil as affected by land use, soil pH and N fertilization, Soil Biology and Biochemistry, 26, p1613-1622.
[https://doi.org/10.1016/0038-0717(94)90313-1]

- IPCC, (2007), Climate change 2007: the physical science basis, contribution of working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge (UK), Cambridge University Press, 2007, p996, ISBN 978-0-521-88009-1.
- Jones, H.A., Nedwell, D.B., (2006), Methane emission and methane oxidation in land-fill cover soil, FEMS Microbiology Letters, 102, p185-195.
-
Jugnia, L-B., Cabral, A.R., Greer, C.W., (2008), Biotic methane oxidation within an instrumented experimental landfill cover, Ecological Engineering, 33, p102-109.
[https://doi.org/10.1016/j.ecoleng.2008.02.003]

-
Keller, J.K., Bauers, A.K., Bridgham, S.D., Kellogg, L.E., Iversen, C.M., (2006), Nutrient control of microbial carbon cycling along an ombrotrophic-minerotrophic peatland gradient, Journal of Biophysical Research Biogeosciences, 111, p1-14.
[https://doi.org/10.1029/2005jg000152]

-
Kettunen, R.H., Einola, J.M., Rintala, J.A., (2006), Landfill methane oxidation in engineered soil columns at low temperature, Water, Air, and Soil Pollution, 177, p313-334.
[https://doi.org/10.1007/s11270-006-9176-0]

- Kightley, D., Nedwell, D.B., Cooper, M., (1995), Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms, Applied and Environmental Microbiology, 61, p592-601.
-
Knowles, R., (2005), Denitrifiers associated with methanotrophs and their potential impact on the nitrogen cycle, Ecological Engineering, 24, p441-446.
[https://doi.org/10.1016/j.ecoleng.2005.01.001]

- Lee, S.-W., (2008), Microbial mitigation of greenhouse gas emissions from landfill cover soils, PhD dissertation, University of Michigan, USA.
-
Lee, S.-W., Im, J., DiSpirito, A.A., Bodrossy, L., Barcelona, M.J., Semrau, J.D., (2009), Effect of nutrient and selective inhibitor amendments on methane oxidation, nitrous oxide production, and key gene presence and expression in landfill cover soils: characterization of the role of methanotrophs, nitrifiers, and denitrifiers, Applied Microbiology and Biotechnology, 85, p389-403.
[https://doi.org/10.1007/s00253-009-2238-7]

-
Maurice, C., Lagerkvist, A., (2003), LFG emission measurements in cold climatic conditions: seasonal variations and methane emissions mitigation, Cold Regions Science and Technology, 36, p37-46.
[https://doi.org/10.1016/s0165-232x(02)00094-0]

- Mer, J.L., Roger, P., (2001), Production, oxidation, emission and consumption of methane by soils: A review, European Journal of Soil Biology, 37, p25-50.
-
Mor, S., De Visscher, A., Ravindra, K., Dahiya, R.P., Chandra, A., Cleemput, O.V., (2006), Induction of enhanced methane oxidation in compost: Temperature and moisture response, Waste Management, 26, p381-388.
[https://doi.org/10.1016/j.wasman.2005.11.005]

- Mosier, A., Wassmann, R., Verchot, L., King, J., PALM, C., (2004), Methane and nitrogen oxide fluxes in tropical agricultural soils: Source, sinks and mechanisms. environment, Development and Sustainability, 6, p11-49.
-
Park, J.-R., Moon, S., Ahn, Y.M., Kim, J.Y., Nam, K., (2005), Determination of environmental factors influencing methane oxidation in a sandy landfill cover soil, Environmental Technology, 26, p93-102.
[https://doi.org/10.1080/09593332608618586]

-
Park, S., Brown, K.W., Thomas, J.C., (2002), The effect of various environmental and design parameters on methane oxidation in a model biofilter, Waste Management and Research, 20, p434-444.
[https://doi.org/10.1177/0734242x0202000507]

- Pawlowska, M., Stepniewski, W., (2006), An influence of methane concentration on the methanotrophic activity of a model landfill cover, Ecological Engineering, 26, p392-395.
- Qingxian, G., Wupeng, D., Shiqing, L., Zhigang, Z., Enchen, Z., Jianguo, W., Zhenhai, R., (2007), Methane emission from municipal solid waste treatments in China, Advances in Climate Change Research, 3, p70-74.
-
Reay, D.S., Nedwell, D.B., (2004), Methane oxidation in temperate soils: effects of inorganic N, Soil Biology and Biochemistry, 36, p2059-2065.
[https://doi.org/10.1016/j.soilbio.2004.06.002]

-
Ritzkowski, M., Stegmann, R., (2007), Controlling greenhouse gas emissions through landfill in situ aeration, International Journal of Greenhouse Gas Control, 1, p281-288.
[https://doi.org/10.1016/s1750-5836(07)00029-1]

-
Saari, A., Rinnan, R., Martikainen, P.J., (2004), Methane oxidation in boreal forest soils: kinetics and sensitivity to pH and ammonium, Soil Biology and Biochemistry, 36, p1037-1046.
[https://doi.org/10.1016/j.soilbio.2004.01.018]

- Scheutz, C., Bogner, J., (2003), Comparative oxidation and net emissions of CH4 and selected non-methane organic compounds in landfill cover soils, Environmental Science and Technology, 37, p5143-5149.
-
Scheutz, C., Kjeldsen, P., (2004), Environmental factors influencing attenuation of methane and hydrochlorofluorocarbons in landfill cover soils, Journal of Environmental Quality, 33, p72-79.
[https://doi.org/10.2134/jeq2004.0072]

- Shangari, S.G., Agamuthu, P., (2012), Enhancing methane oxidation in landfill cover using brewery spent grain as Biocover, Malaysian Journal of Science, 31, p91-97.
-
Stern, J.C., Chanton, J., Abichou, T., Powelson, D., Yuan, L., Escoriza, S., Bogner, J., (2007), Use of biologically active cover to reduce landfill methane emissions and enhance methane oxidation, Waste Management, 27, p1248-1258.
[https://doi.org/10.1016/j.wasman.2006.07.018]

-
Streese, J., Stegmann, R., (2003), Microbial oxidation of methane from old landfills in biofilters, Waste Management, 23, p573-580.
[https://doi.org/10.1016/s0956-053x(03)00097-7]

- Tanthachoon, N., Chiemchaisri, C., Chiemchaisri, W., (2007), Alternative Approach for Encouraging Methane Oxidation in Compost Based Landfill Cover Layer with Vegetation, In: Proceedings of the International Conference on Sustainable Solid Waste Management, 5-7 September, p202-209, Chennai, India.
- Tecle, D., Lee, J., Hasan, S., (2008), Quantitative analysis of physical and geotechnical factors affecting methane emission in municipal solid waste landfill, Environmental Geology, 56, p1135-1143.
-
Visvanathan, C., Pokhrel, D., Cheimchaisri, W., Hettiaratchi, J.P.A., Wu, J.S., (1999), Methanotrophic activities in tropical landfill cover soils: effect of temperature, moisture content and methane concentration, Waste Management and Research, 17, p313-323.
[https://doi.org/10.1034/j.1399-3070.1999.00052.x]

-
Wang, Y., Wu, W., Ding, Y., Liu, W., Perera, A., Chen, Y., Devare, M., (2008), Methane oxidation activity and bacterial community composition in a simulated landfill cover soil is influenced by the growth of Chenopodium album L, Soil Biology and Biochemistry, 40, p2452-2459.
[https://doi.org/10.1016/j.soilbio.2008.06.009]

-
Wang, Z.-P., Ineson, P., (2003), Methane oxidation in a temperate coniferous forest soil: effects of inorganic N, Soil Biology and Biochemistry, 35, p427-433.
[https://doi.org/10.1016/s0038-0717(02)00294-8]

-
Whittenbury, R., Phillips, K.C., Wilkinson, J.F., (1970), Enrichment, Isolation and Some Properties of Methane-utilizing Bacteria, Journal of General Microbiology, 61, p205-218.
[https://doi.org/10.1099/00221287-61-2-205]

- Willam, G.M., Zobell, C., (1949), The occurrence and characteristic of methane of oxidizing bacteria in marine sediments, 58, p463-473.
- Wilshusen, J.H., Hettiaratchi, J.P.A., Visscher, A.D., Saint-Fort, R., (2004a), Methane oxidation and formation of EPS in compost: effect of oxygen concentration, Environmental Pollution, 129, p305-314.
- Wilshusen, J.H., Hettiaratchi, J.P.A., Stein, V.B., (2004b), Long-term behavior of passively aerated compost methanotrophic biofilter columns, Waste Management, 24, p643-653.