
Effects of Long-term Exposure to Black Carbon Particles on Growth and Gas Exchange Rates of Fagus crenata, Castanopsis sieboldii, Larix kaempferi and Cryptomeria japonica Seedlings
Abstract
To clarify the effects of black carbon (BC) particles on growth and gas exchange rates of Asian forest tree species, the seedlings of Fagus crenata, Castanopsis sieboldii, Larix kaempferi and Cryptomeria japonica were exposed to BC particles with sub-micron size for two growing seasons from 1 June 2009 to 11 November 2010. The BC particles deposited after the exposure to BC were observed on the foliar surface of the 4 tree species. At the end of the experiment, the amount of BC accumulated on the foliar surface after the exposure to BC aerosols were 0.13, 0.69, 0.32 and 0.58 mg C m-2 total leaf area in F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings, respectively. In August 2010, the exposure to BC particles did not significantly affect net photosynthetic rate under any light intensity, stomatal diffusive conductance to water vapour (gs), stomatal limitation of photosynthesis, response of gs to increase in vapour pressure deficit and leaf temperature under light saturated condition in the leaves or needles of the seedlings. These results suggest that the BC particles deposited on the foliar surface did not reduce net photosynthesis by shading, did not increase leaf temperature by absorption of irradiation light, and did not induce plugging of stomata in the leaves or needles of the seedlings. There were no significant effects of BC particles on the increments of plant height and stem base diameter during the experimental period and the whole-plant dry mass at the end of the experiment. These results indicate that the exposure to BC particles with sub-micron size for two growing seasons did not significantly affect the growth and leaf or needle gas exchange rates of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings.
Keywords:
Forest tree species, Black carbon particles, Exposure, Growth, Gas exchange rates, Stomatal diffusive conductance1. INTRODUCTION
Recently, East Asian countries face the problem of transboundary air pollution including aerosol particles and its effects on vegetation (Izuta and Funada, 2010). The aerosol particles present in the atmosphere vary in size from a few nm to a few hundred μm in diameter (Chin et al., 2007). The sub-micron sized particles are originated mainly from the anthropogenic sources (e.g. combustion) (Kasahara, 2004; Colvile, 2002). Because the particles with sub-micron size deposit quite slowly to terrestrial surfaces, sub-micron sized particles have relatively long atmospheric lifetimes in the absence of precipitation and are transboundary air pollutant (Fowler, 2002; Colvile, 2002). Furthermore, the deposition rates onto forests are substantially larger than those onto short vegetation (Matsuda et al., 2010; Petroff et al., 2008; Fowler, 2002; Ruijgrok et al., 1997). Therefore, it is necessary to clarify the effects of sub-micron sized aerosol particles on growth and physiological functions of East Asian forest tree species.
Limited information on the effects of sub-micron sized aerosol particles on plants is available at the present time. Burkhardt et al. (2001) reported that the exposure to NaNO3 particles (0.5-1.0 μm in diameter) for 3 to 4 hours increased transpiration rate in the leaves of Sambucus nigra. Hirano et al. (1995, 1991) reported that the exposure of Cucumis sativus and Phaseolus vulguris to black carbon (BC) particles within 3 min. reduced net photosynthetic rate by shading, and increased leaf temperature by absorption of irradiation light. These authors used BC with primary particle sizes of 0.03-0.2 μm(SEM-based sizes), and based on the fluidized bed-type aerosol generator they used, their system dispersed particles over a size range from sub-μm to few ten μm. However, there is no information on the long-term effects of sub-micron sized aerosol particles on growth and physiological functions such as photosynthesis of forest tree species.
The BC is a component of fine particulate matter. The main sources of BC are combustion of fossil fuels and biomass burning (WHO, 2012). The major source regions of BC are developing nations in the tropics and East Asia (Ramanathan and Carmichael, 2008). In Asian region, furthermore, it is predicted that BC emissions will increase (Ohara et al., 2007). To evaluate the effects of anthropogenic aerosol particles such as BC particles on forest ecosystems in East Asia, in the present study, we investigated the effects of BC particles with sub-micron size on growth and physiological functions of forest tree species native to East Asia.
2. MATERIALS AND METHODS
2. 1 Plant Materials
On 8 May 2009, 3-year-old seedlings of Fagus crenata (deciduous broad-leaved tree), 2-year-old seedlings of Castanopsis sieboldii (evergreen broad-leaved tree), 1-year-old seedlings of Larix kaempferi (deciduous conifer) and 1-year-old seedlings of Cryptomeria japonica (evergreen conifer) were individually planted in 2 L pots filled with Kanuma pumice soil. During the experimental period from 1 June 2009 to 11 November 2010, the seedlings were grown in 6 phytotron chambers (around 2×2×2 m glasshouse with an air-conditioner system) located at Tokyo University of Agriculture and Technology (Fuchu, Tokyo, Japan). In the chambers, the seedlings received natural day length, and washing out of any particles deposited on the foliar surface was avoided. For each tree species, 10 seedlings were assigned to each chamber, for a total of 60 seedlings per species. The whole-plant dry mass, plant height and stem base diameter of the seedlings at the beginning of the experiment were 11.7±3.3 g, 31.0±3.7 cm and 6.6±0.8 mm for F. crenata, 13.0±2.9 g, 35.0±3.4 cm and 4.7±0.5 mm for C. sieboldii, 2.9±0.8 g, 19.9±2.9 cm and 4.1±0.5 mm for L. kaempferi, and 2.2±0.9 g, 19.2±3.1 cm and 3.3±0.6 mm for C. japonica, respectively.
In the chambers, air temperature and relative air humidity were maintained at 25.0±1.0/18.0±1.0°C (6: 00-18:00/18:00-6:00) and 70±5%, respectively, from 1 June to 7 December 2009 and from 17 April to 11 November 2010. From 7 January to 19 March 2010, the seedlings were grown under field condition to induce winter dormancy. During the winter dormancy period, to avoid washing out of any particles deposited on the leaf or needle surface, all the seedlings were protected from natural precipitation and snowfall by a transparent polyvinylchloride roof on rainy and snowy days. For approximately one month before and after the winter dormancy period, to acclimatize the seedlings to field condition, air temperature and relative air humidity in the chambers were maintained at 17.0±1.0/12.0±1.0°C (6:00-18:00/18:00-6:00) and 60±5%, respectively. During the growth period, all the seedlings were irrigated as necessary with tap water and fertilized at the two-week intervals with 200 mL of liquid fertilizer (HYPONeX, N : P : K=6% : 10% : 5%, Hyponex Japan Co. Ltd., Japan) diluted 2,000 times.
2. 2 Black Carbon Exposure
The seedlings were exposed to black carbon (BC) particles with sub-micron size generated by an aerosol generator system newly developed for this study. The system is based on an electrostatic-spray (Lenggoro et al., 2002) running for 10 min. and an ultrasonic nebulizer (Wang et al., 2008) running for 5 min. using suspension of BC particles. The source of BC is a combustion-derived nanopowders (suspension) with primary particle size around 30 nm, and mainly consisting of elemental carbon. The size distributions of generated and suspended aerosols in the dry condition (i.e. the solid BC particles) were measured by a realtime technique based on a differential mobility analyzer (DMA) method (Wang et al., 2008; Lenggoro et al., 2002). The measured mean size of BC particles was between 100-300 nm. This result indicates that the generated (dry) aerosols are formed by the aggregation of BC particles having primary particle size of around 30 nm. From 13 June 2009 to 6 January 2010 and from 21 March 2010 to 12 June 2010, the half of the seedlings were exposed to BC particles every two days within 6:00-9:00 (BC treatment), and the other half of the seedlings were not exposed to the particles (control treatment). Because the deposition efficiency of submicro-meter sized aerosols is low, the BC particles were driven against the seedlings under windless condition in the present study. The windless condition was achieved by stopping the air circulation and air-conditioner system. To avoid the adverse effect of high air temperature on the seedlings during the stopping the system, it is necessary to conduct the BC exposure during the time when ambient air temperature and sunlight intensity were low. On the other hand, it was reported that aerosol particles has plugging effect on stomata (Hirano et al., 1995; Flückiger et al., 1979; Ricks and Williams, 1974). Because light stimulates stomatal opening (Roelfsema and Hedrich, 2005), exposure to BC was conducted within 6:00-9:00 to clarify the plugging effect of sub-micron sized BC particles on stomata. From 7 January to 20 March 2010, BC exposure could not be conducted, because the seedlings were not enclosed during winter dormancy period as mentioned above. At the end of the first growing season, the amount of BC deposited on foliar surface (MBC) of the seedlings were lower than that observed in the field which may include particles larger than few micrometers (e.g. Matsuda et al., 2012). To achieve the greater MBC, therefore, the exposure time was extended to 5 times and the seedlings grown in the BC treatment were exposed to BC particles every day within 6:00-9:00 from 13 June to 31 October 2010. In each treatment, 3 chamber replications for a total of 6 chambers were used.
2. 3 Measurements of Plant Growth
On 1 June 2009 and 1 November 2010, plant height and stem base diameter of all the seedlings were measured. Increments of plant height and stem base diameter during the experimental period were differences between plant height and stem base diameter on 1 June 2009 and those on 1 November 2010. On 12-17 November 2010, all the seedlings were harvested. The harvested seedlings were separated into the plant organs. The separated plant organs of the harvested seedlings were dried at 80°C in an oven for 1 week and weighed.
2. 4 Observation of BC Particles Deposited on the Foliar Surface
On 12-17 November 2010, the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings, which were exposed to BC in the growth chambers, were harvested to observe the BC particles deposited on the surface by field-emission scanning electron microscopy (FE-SEM). The harvested leaves and needles were air-dried in desiccators, fixed onto specimen stubs for FE-SEM and coated with platinumpalladium using a sputter coater or with platinum by vacuum evaporation. The foliar surface was observed under field-emission scanning electron microscopes (S-4800, HITACHI) at 0.5 to 2.5 kV.
2. 5 Quantification of BC Particles Deposited on Foliar Surface
On 12-17 November 2010, the leaves or needles of the harvested seedlings were washed with distilled water followed by the washing with chloroform for 20 seconds. To quantify MBC, the particles suspended in the distilled water and chloroform were then collected on a quartz fiber filter (QR-100, Advantec MFS, Inc., Japan) by filtration. Filtration efficiency of BC particles suspended in water was considered to be more than 80% (Matsuda et al., 2012) and that in chloroform was confirmed as more than 90% according to the method of Matsuda et al. (2012) (data not shown). To determine the amounts of BC particles collected on the quartz fiber filter, the absorbance at 580 nm of the quartz fiber filters (A580) were measured by a spectrophotometer with an integrating sphere (U-4100, Hitachi High Technologies Corp., Japan). In the quantification of BC, a calibration line was made between A580 of the filter and amount of BC collected on the filters measured by the thermal optical reflectance method using a DRI OC/EC carbon analyzer (Model 2001A). The MBC was expressed on the basis of total leaf surface area. Leaf area of F. crenata and C. sieboldii seedlings was measured with an area meter (Hayashi Denko Co. Ltd., Japan). Leaf area of L. kaempferi seedlings was determined by image analysis software (LIA32, http://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/index.html). Needle surface area of C. japonica was determined by the method of Sase et al. (1998).
2. 6 Leaf Gas Exchange Rates
To determine leaf or needle gas exchange rates, 3 seedlings per treatment-chamber combination were randomly selected for the analyses (9 determinations per treatment). Gas exchange rates of the leaves or needles of F. crenata, C. sieboldii (current-year leaves), L. kaempferi and C. japonica (current-year needles) were measured from 25 August to 4 September 2010. The measurements were performed using an infrared gas analyzer system (LI-6400, Li-Cor Inc., NE, U.S.A). Light-saturated net photosynthetic rate (Asat), stomatal diffusive conductance to H2O(gs) and leaf temperature (Tleaf) were determined at air temperature of 25.0±0.1 °C, 380 μmol CO2 mol-1, relative air humidity of 70±5% and photosynthetic photon flux density (PPFD) of 1500 μmol m-2 s-1. To obtain photosynthetic light-response curve, net photosynthetic rates (A) were measured under different PPFD at the foliar surface ranging from 1500 to 0 μmol m-2 s-1. On 16-24 August 2010, stomatal limitation of photosynthesis (Ls) and response of gs to increase in vapour pressure deficit (VPD) were measured in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica. To examine the relative limitation of net photosynthetic rate by stomatal diffusive resistance, the response of A to change in the intercellular CO2 concentration (Ci) was measured by varying inlet CO2 concentration from 0 to 500 μmol CO2 mol-1. Carboxylation efficiency (CE) was calculated from the linear relationship between A and Ci ranging from 0 to 400 μmol CO2 mol-1. The Ls was calculated by the following function:
Ls(%)=[(Ao-A380)/Ao]×100
where Ao is the A estimated from CE and the assumption that Ci was equal to 380 μmol CO2 mol-1, and A380 is the A measured at inlet CO2 concentration of 380 μmol CO2 mol-1. To examine the stomatal response to increase in VPD (dgs/dVPD), the reduction rate of gs with increase in leaf-to-air VPD from 1.5 to 3.5 kPa was measured by varying inlet H2O concentration. The dgs/dVPD was calculated as slope of the regression line between vapour pressure deficit and relative value of gs (gs determined at 1.5 kPa VPD was defined as 1.0).
2. 7 Statistical Analyses
Statistical analyses were performed with the IBM® SPSS® Advanced Statistics 19. To identify significant difference between the mean of 3 chamber replications in the control treatment and that in the black carbon treatment, paired t-test was performed (p<0.05).
3. RESULTS AND DISCUSSION
The FE-SEM images of foliar surface of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings grown in black carbon (BC) treatment were shown in Fig. 1. The BC particles deposited after the BC exposure were observed in all the tree species. The amount of BC particles deposited on the foliar surface (MBC) of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings was indicated in Fig. 2. In all the tree species, the MBC of the seedlings grown in the BC treatment was higher than that of the seedlings grown in the control treatment. These results indicate that the aerosol exposure chamber and generation system of BC particles with sub-micron size adopted in the present study is useful for relatively long-term experimental study on the effects of aerosol particles on the seedlings of forest tree species.
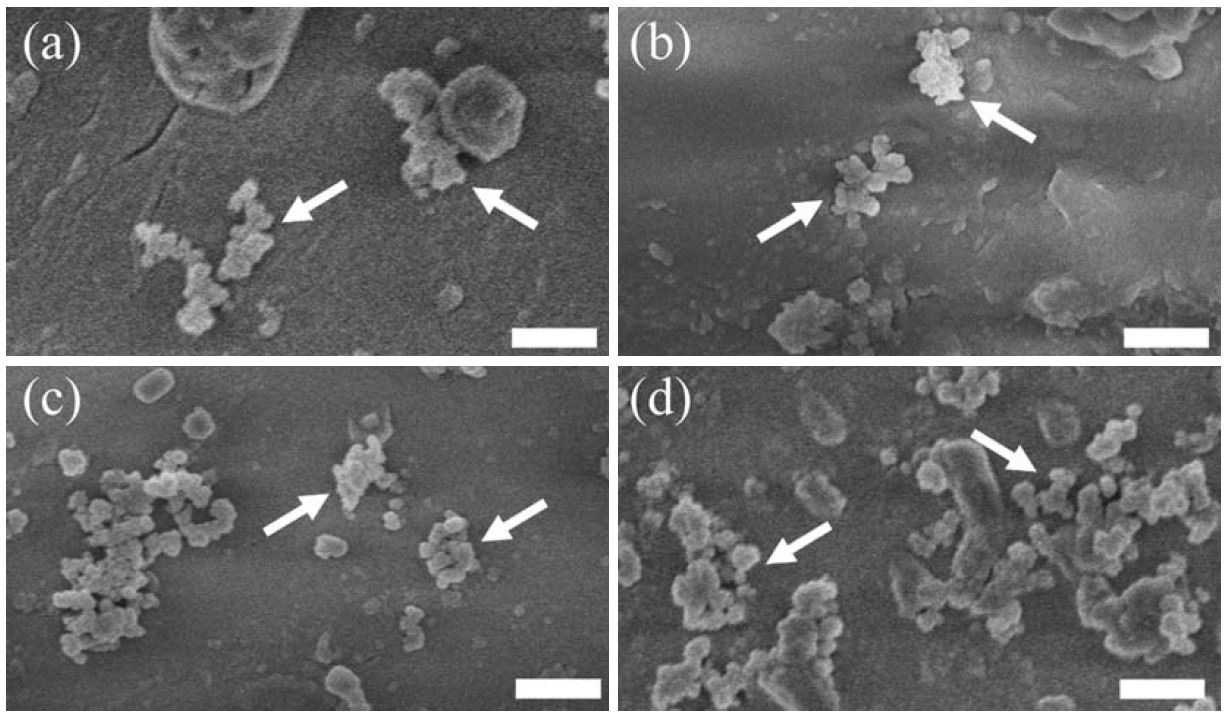
Field-emission scanning electron micrographs showing black carbon (BC) particles deposited on the leaves or needles of (a) F. crenata, (b) C. sieboldii, (c) L. kaempferi and (d) C. japonica seedlings grown in the BC treatment. Arrows indicate the BC particles originated from the BC exposure. Bars=500 nm.
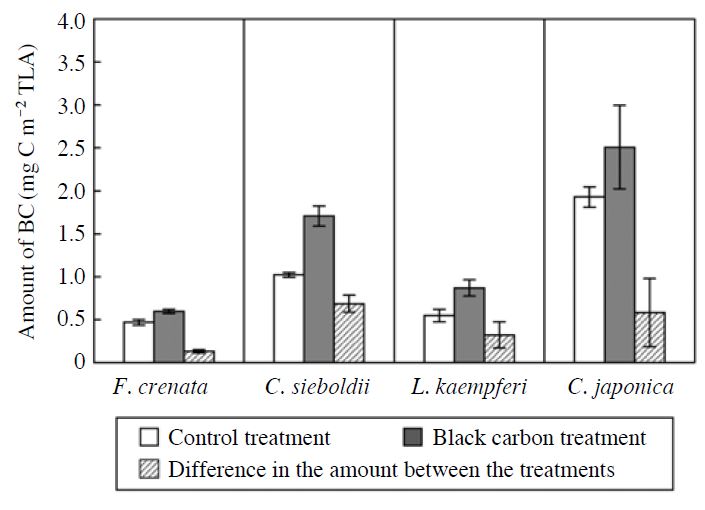
Amount of black carbon (BC) particles deposited on the surface of the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in November 2010. The amount of BC particles was expressed on the basis of total leaf area (TLA). Each value shows the mean of 3 chamber replications, and the standard deviation is given by vertical bar.
In the present study, the degree of increase in MBC by the exposure to BC particles was little as compared with MBC in the control treatment (Fig. 2). Because the seedlings were grown in the glasshouse chambers, washing out of any particles deposited on the leaf or needle surface was avoided. Furthermore, the upper direction wind was always blowing (at most 0.5 m s-1) in the chamber except for the exposure time, resulting in constant deposition of ambient BC, including both fine (below 1 μm) and coarse (above 1 μm) particles, onto the foliar surface of the seedlings. As a result, weight-based MBC of the seedlings grown in the control treatment was relatively high (Fig. 2). On the other hand, difference in MBC between the control treatment and the BC treatment can be considered as the MBC accumulated by the exposure to BC particles. The MBC accumulated by the exposure to BC particles consisted of mainly fine particles. As compared with MBC in the control treatment, therefore, the weight-based MBC accumulated by the exposure to BC particles was relatively low.
It is important for development of the generic dry deposition model to understand the relationship between generated amount of BC and MBC of the seedlings. Because a “direct” deposition technique was used in the chamber, it is difficult to compare between the number concentrations of BC (solid) particles generated from the nozzle with those of suspended (non-deposited) in the chamber and deposited particles on the plants, even a real-time apparatus (such as a laser-based submicron-particle counter) was introduced in the chamber. Therefore, it is difficult to consider the relationship between generated amount of BC and MBC to discuss the deposition process of BC in the chamber. However, the species difference in MBC was obvious especially between deciduous and evergreen tree species. The higher MBC in evergreen tree species (C. sieboldii and C. japonica) as compared with that in deciduous tree species (F. crenata and L. kaempferi) (Fig. 2) can be attributed to longer leaf longevity in evergreen tree species. On the other hand, within the both evergreen and deciduous species, the MBC in coniferous tree species was higher than that in the broad-leaved tree species (Fig. 2). This result was consistent with those reported by Beckett et al. (2000), Freer-Smith et al. (2005) and Hwang et al. (2011). Such greater capture efficiency of particles by shoots in coniferous tree species as compared with that in broad-leaved tree species can be explained by complex structure of shoot and smaller leaves (Beckett et al., 2000).
The effects of BC particles on the response of net photosynthetic rates to photosynthetic photon flux density (PPFD) (A-light curve) in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in August 2010 were indicated in Fig. 3. The exposure to BC particles did not significantly affect net photosynthetic rates in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings under any PPFD. This result suggests that the BC particles deposited on the foliar surface did not significantly reduce net photosynthesis of the seedlings by shading. Table 1 indicates the effects of BC particles on net photosynthetic rate (Asat) and stomatal diffusive conductance to water vapour (gs) under light-saturated condition, stomatal limitation of photosynthesis (Ls), response of gs to increase in vapour pressure deficit (dgs/dVPD) and difference in temperature between leaf and air (Tleaf - Tair) in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in August 2010. There was no significant effect of BC particles on the Tleaf - Tair in the leaves or needles under light-saturated condition. This result suggests that the BC particles deposited on the foliar surface did not increase leaf temperature by absorption of irradiation light. There were no significant effects of BC particles on the gs, Ls and dgs/dVPD in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings. These results suggest that the BC particles deposited on the leaves or needles did not induce plugging of stomata in F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings.

Effects of black carbon particles on net photosynthetic rate (Asat), stomatal diffusive conductance to water vapour (gs) under light-saturated condition, stomatal limitaion of photosynthesis (Ls), response of gs to increase in vapour pressure deficit (dgs/dVPD) and difference in temperature between leaf and air (Tleaf-Tair) in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in August 2010.
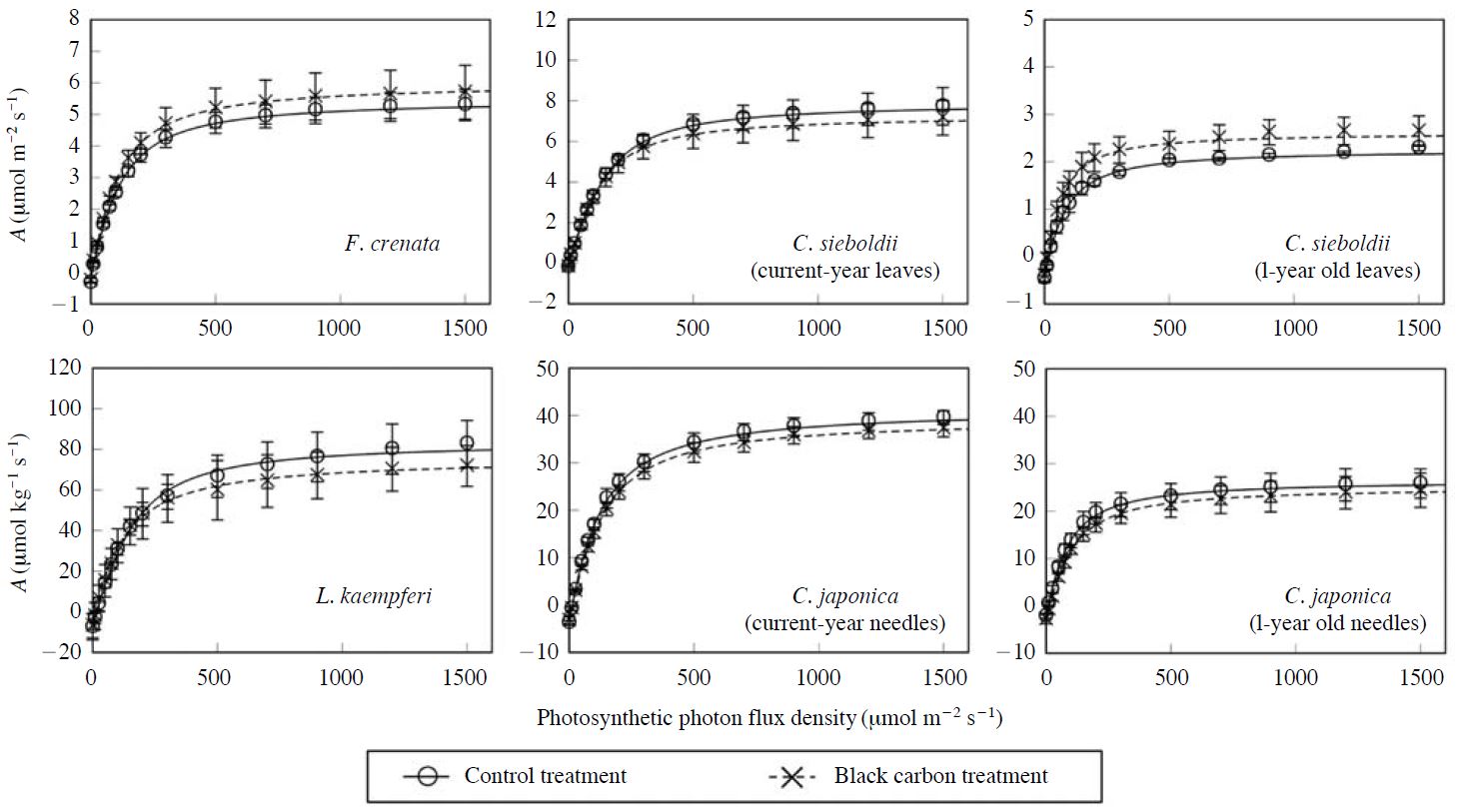
Effects of black carbon particles on the response of net photosynthetic rates (A) to photosynthetic photon flux density in the leaves or needles of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in August 2010. Each value shows the mean of 3 chamber replications, and the standard deviation is given by vertical bar. The regression analysis was performed using non-rectangular hyperbolic function.
The effects of BC particles on the increments of plant height and stem base diameter of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings during the experimental period were indicated in Fig. 4. There were no significant effects of BC particles on the increments of plant height and stem base diameter of the seedlings. The effects of BC particles on the wholeplant dry mass of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in November 2010 were shown in Fig. 5. The exposure to BC particles did not significantly affect the whole-plant dry mass of the seedlings at the end of the experiment. These results indicate that the exposure to BC particles for two growing seasons did not significantly affect the growth of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings. The MBC accumulated by the exposure to BC particles were 0.13, 0.69, 0.32 and 0.58 mg C m-2 total leaf area (TLA) in F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings, respectively (Fig. 2). In Cucumis sativus and Phaseolus vulguris, net photosynthetic rate was reduced by shading effect of MBC at >320 mg C m-2 projected leaf area (PLA) (i.e. 160 mg C m-2 TLA) (Hirano et al., 1991). Using BC generated by the fluidized bed-type generator, with size range from sub-μm to few ten μm, Hirano et al. (1995) reported that leaf temperature of C. sativus and P. vulguris was increased due to the absorption of irradiation light by MBC at >400 mg C m-2 PLA (i.e. 200 mg C m-2 TLA). With the existence of particle at few ten μm range, these weight-based MBC (Hirano et al., 1995) were quite high as compared with those observed in the present study (Fig. 2). As a consequence, it is possible that the reduction in net photosynthetic rate of the tree species was not observed in the present study, and hence the growth of the seedlings was not reduced by the exposure to BC particles. To evaluate the effects of BC particles on growth and leaf or needle gas exchange rates of forest tree species in the field, it is necessary to determine whether or not the amount of BC deposited on the foliar surface of forest trees grown in the field is higher than the amount observed in the present study.
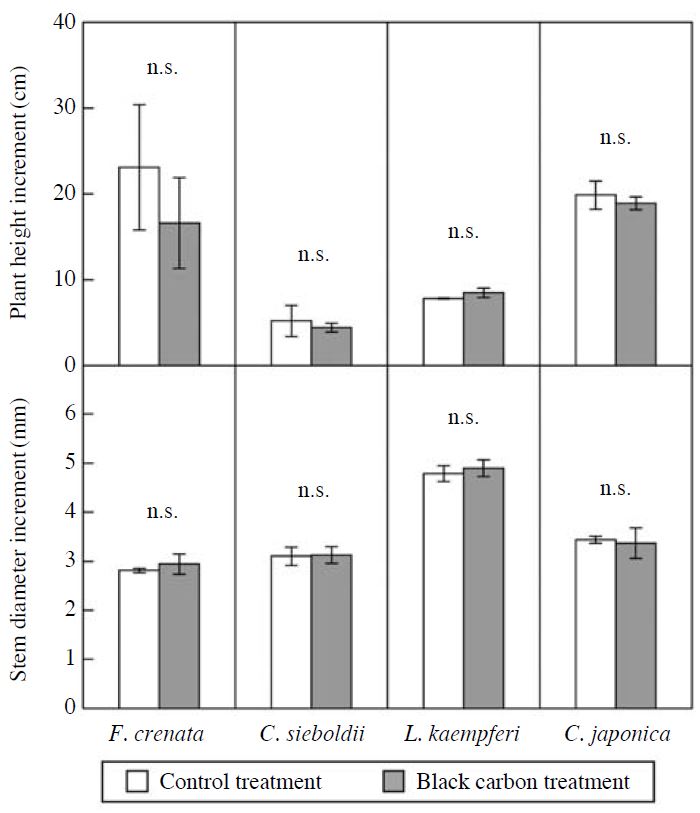
Effects of black carbon particles on the increments of plant height (top) and stem base diameter (bottom) of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings during the experimental period. Each value shows the mean of 3 chamber replications, and the standard deviation is given by vertical bar. n.s.=not significant (paired t-test).
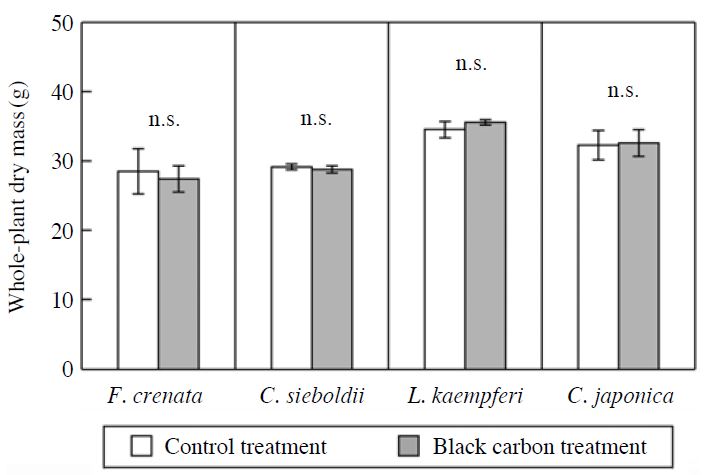
Effects of black carbon particles on the whole-plant dry mass of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings in November 2010. Each value shows the mean of 3 chamber replications, and the standard deviation is given by vertical bar. n.s.=not significant (paired t-test).
Recently, Matsuda et al. (2012) reported that the amount of deposition of elemental carbon from the atmosphere to tropical forest in Thailand during the leafy season (i.e. growing season) in 2010 was estimated to be 0.34 mg C m-2 day-1. This value corresponded to MBC of 8.9 mg C m-2 TLA during the season, assuming that the leaf area index of the forest and the number of days during the season were 3.5 and 183, respectively (Matsuda et al., 2012). The MBC reported by Hirano et al. (1995, 1991) was very high as compared with that observed in the field and, thus, not realistic. To clarify the effects of BC particles on the growth and physiological function of forest tree species in the field, it is necessary to clarify the effects of BC particles deposited on the foliar surface at realistic level. In the present study, therefore, we tried to make the MBC at ambient level, resulting in lower MBC than that reported by Hirano et al. (1995, 1991). However, the MBC in the present study (start in 2009) was also lower than that observed in the forest as reported in a recent study (Matsuda et al., 2012). Because the objective of the present study is to clarify the effect of sub-micron sized (long-range transport) aerosol particles, the seedlings were exposed to sub-micron sized BC particles. Therefore, the MBC accumulated by the exposure to BC particles consisted of mostly sub-micron range particles with light mass per particle. On the other hand, in the field, the MBC was not sorted out by particle size and consisted of not only fine particle (i.e. sub-micron size) but also coarse particle with relatively heavy mass per particle. As a result, the weight-basis MBC in the present study was lower than that in the field. Therefore, it is necessary to sort out the weight of particles versus their sizes, and to clarify the relationship between field-observed amount and size range of BC deposited on the foliar surface and growth and physiological functions of forest tree species.
4. CONCLUSIONS
The exposure to BC particles with sub-micron size for two growing seasons dose not significantly affect the growth and leaf or needle gas exchange rates of F. crenata, C. sieboldii, L. kaempferi and C. japonica seedlings. To evaluate the effects of BC particles on forest tree species grown under field conditions, further study is needed to clarify the relationship between the amount of BC deposited on the foliar surface and the degree of the negative effects of BC on forest tree species.
Acknowledgments
The authors are greatly indebted to Dr. Tsumugu Totsuka and Dr. Takejiro Takamatsu for their invaluable advices. The authors also acknowledge Prof. Hidehiro Kamiya, Mr. Shin-ichi Sagawa, Mr. Masao Gen, Ms. Fong Zyin Lim, Mr. Akito Seki and Mr. Peiran Li (Tokyo University of Agriculture and Technology) for their valuable suggestion and technical support. This research was funded by Grant-in-Aid for Scientific Research on Innovative Areas (No. 20120009, No. 20120010) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
-
Beckett, K.P., Freer-Smith, P.H., Taylor, G., (2000), Particulate pollution capture by urban trees: effect of species and windspeed, Global Change Biology, 6, p995-1003.
[https://doi.org/10.1046/j.1365-2486.2000.00376.x]

-
Burkhardt, J., Kaiser, H., Kappen, L., Goldbach, H.E., (2001), The possible role of aerosols on stomatal conductivity for water vapour, Basic and Applied Ecology, 2, p351-364.
[https://doi.org/10.1078/1439-1791-00062]

-
Chin, M., Diehl, T., Ginoux, P., Malm, W., (2007), Intercontinental transport of pollution and dust aerosols: Implications for regional air quality, Atmospheric Chemistry and Physics, 7, p5501-5517.
[https://doi.org/10.5194/acp-7-5501-2007]

- Colvile, R.N., (2002), Emissions, dispersion and atmospheric transformation, In Air Pollution and Plant Life, second edition (Bell, J.N.B. and Treshow, M. Eds), John Wiley & Sons, Ltd, England, p23-42.
-
Flückiger, W., Oertli, J., Flückiger, H., (1979), Relationship between stomatal diffusive resistance and various applied particle size on leaf surfaces, Zeitschrift für Pflanzenphysiologie, 91, p173-175.
[https://doi.org/10.1016/s0044-328x(79)80091-4]

- Fowler, D., (2002), Pollutant deposition and uptake by vegetation, In Air Pollution and Plant Life, second edition (Bell, J.N.B. and Treshow, M. Eds), John Wiley & Sons, Ltd, England, p43-68.
- Freer-Smith, P.H., Beckett, K.P., Taylor, G., (2005), Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides×trichocarpa ‘Beaupré’, Pinus nigra and ×Cupressocyparis leylandii for coarse, fine and ultrafine particles in the urban environment, Environmental Pollution, 133, p157-167.
-
Hirano, T., Kiyota, M., Aiga, I., (1991), The effects of dust by covering and plugging stomata and by increasing leaf temperature on photosynthetic rate of plant leaves, Journal of Agricultural Meteorology, 46, p215-222, (in Japanese with English summary).
[https://doi.org/10.2480/agrmet.46.215]

-
Hirano, T., Kiyota, M., Aiga, I., (1995), Physical effects of dust on leaf physiology of cucumber and kidney bean plants, Environmental Pollution, 89, p255-261.
[https://doi.org/10.1016/0269-7491(94)00075-o]

-
Hwang, H.-J., Yook, S.-J., Ahn, K.-H., (2011), Experimental investigation of submicron and ultrafine soot particle removal by tree leaves, Atmospheric Environment, 45, p6987-6994.
[https://doi.org/10.1016/j.atmosenv.2011.09.019]

- Izuta, T., Funada, R., (2010), Toward the clarification of the effects of aerosol on forests in East Asia, Hoppo Ringyo, 62, p5-8, (in Japanese).
- Kasahara, M., (2004), Source and characteristics of aerosols, In Glossary of Aerosol Science (Edited by Japan Association of Aerosol Science and Technology), p22-23, Kyoto University Press, Japan, (in Japanese).
-
Lenggoro, I.W., Xia, B., Okuyama, K., (2002), Sizing of colloidal nanoparticles by electrospray and differential mobility analyzer methods, Langmuir, 18, p4584-4591.
[https://doi.org/10.1021/la015667t]

-
Matsuda, K., Fujimura, Y., Hayashi, K., Takahashi, A., Nakaya, K., (2010), Deposition velocity of PM2.5 sulfate in the summer above a deciduous forest in central Japan, Atmospheric Environment, 44, p4582-4587.
[https://doi.org/10.1016/j.atmosenv.2010.08.015]

-
Matsuda, K., Sase, H., Murao, N., Fukazawa, T., Khoomsub, K., Chanonmuang, P., Visaratana, T., Khummongkol, P., (2012), Dry and wet deposition of elemental carbon on a tropical forest in Thailand, Atmospheric Environment, 54, p282-287.
[https://doi.org/10.1016/j.atmosenv.2012.02.022]

-
Ohara, T., Akimoto, H., Kurokawa, J., Horii, N., Yamaji, K., Yan, X., Hayasaka, T., (2007), An Asian emission inventory of anthropogenic emission sources for the period 1980-2020, Atmospheric Chemistry and Physics Discussions, 7, p6843-6902.
[https://doi.org/10.5194/acpd-7-6843-2007]

-
Petroff, A., Mailliat, A., Amielh, M., Anselmet, F., (2008), Aerosol dry deposition on vegetative canopies. Part II: A new modelling approach and applications, Atmospheric Environment, 42, p3654-3683.
[https://doi.org/10.1016/j.atmosenv.2007.12.060]

-
Ramanathan, V., Carmichael, G., (2008), Global and regional climate changes due to black carbon, Nature Geoscience, 1, p221-227.
[https://doi.org/10.1038/ngeo156]

-
Ricks, G.R., Williams, R.J.H., (1974), Effects of atmospheric pollution on deciduous woodland part 2: Effects of particulate matter upon stomatal diffusion resistance in leaves of Quercus petraea (Mattuschka) Leibl, Environmental Pollution, 6, p87-109.
[https://doi.org/10.1016/0013-9327(74)90026-3]

-
Roelfsema, M.R.G., Hedrich, R., (2005), In the light of stomatal opening: new insights into ‘the Watergate’, New Phytologist, 167, p665-691.
[https://doi.org/10.1111/j.1469-8137.2005.01460.x]

-
Ruijgrok, W., Tieben, H., Eisinga, P., (1997), The dry deposition of particles to a forest canpy: A comparison of model and experimental results, Atmospheric Environment, 31, p399-415.
[https://doi.org/10.1016/s1352-2310(96)00089-1]

-
Sase, H., Takamatsu, T., Yoshida, T., (1998), Variation in amount and elemental composition of epicuticular wax in Japanese cedar (Cryptomeria japonica) leaves associated with natural environmental factors, Canadian Journal of Forest Research, 28, p87-97.
[https://doi.org/10.1139/x97-167]

-
Wang, W.-N., Purwanto, A., Lenggoro, I.W., Okuyama, K., Chang, H., Jang, H.D., (2008), Investigation on the correlations between droplet and particle size distribution in ultrasonic spray pyrolysis, Industrial and Engineering Chemistry Research, 47, p1650-1659.
[https://doi.org/10.1021/ie070821d]

- WHO (World Health Organization), (2012), Health effects of black carbon, WHO Regional Office for Europe, Bonn.