Preliminary Study on the Visualization and Quantification of Elemental Compositions in Individual Microdroplets using Solidification and Synchrotron Radiation Techniques
Abstract
Quantifying the solute composition of a cloud droplet (or a whole droplet) is an important task for understanding formation processes and heating/cooling rates. In this study, a combination of droplet fixation and SR-XRF microprobe analysis was used to visualize and quantify elements in a micro-scale droplet. In this study, we report the preliminary outcome of this experiment. A spherical micro-scale droplet was successfully solidified through exposure to α-cyanoacrylate vapor without affecting its size or shape. An X-ray microprobe system equipped at the beam line 37XU of Super Photon ring 8 GeV (SPring-8) was applied to visualize and quantify the elemental composition in an individual micro-scale droplet. It was possible to reconstruct 2D elemental maps for the K and Cl contained in a microdroplet that was dispensed from the 10-ppm KCl standard solution. Multi-elemental peaks corresponding to X-ray energy were also successfully resolved. Further experiments to determine quantitative measures of elemental mass in individual droplets and high-resolution X-ray microtomography (i.e., 3D elemental distribution) are planned for the future.
Keywords:
Cloud, Individual droplet, Quantification, Elements, Fixation, XRF microprobe1. INTRODUCTION
Clouds are formed at the point of water vapor supersaturation due to the condensation of water vapor on atmospheric particles that serve as cloud condensation nuclei (CCN). The ability of a given particle to serve as a CCN depends on its size, chemical composition, and local supersaturation. After formation, clouds influence the earth radiation budget due to their optical reflectivity and absorptivity. This aerosol indirect climatic effect is broadly defined as the overall process through which aerosols perturb the balance between the Earth and atmospheric radiation as a result of altered cloud albedo and cloud amount. The optical properties of clouds depend on both the in-cloud droplet size distribution and the chemical properties of the cloud droplets. An increase in aerosol number concentration at both urban and background sites tends to decrease average cloud drop size, which enhances cloud albedo (“first indirect effect”) (Twomey, 1977) and can reduce precipitation efficiency (“second indirect effect”) (Albrecht, 1989).
Estimates of aerosol indirect climatic effects are subject to great uncertainty (Intergovernmental Panel on Climate Change (IPCC), 2007) because of the incomplete representation of cloud microphysical processes (Lohmann and Feichter, 2005; Menon et al., 2002; Jones et al., 2001) and chemical characteristics of cloud droplets.
The factors that determine the chemical properties of cloud droplets are the dissolution of soluble gases (Kulmala et al., 1993), partially soluble solutes in the growing droplet (Shulman et al., 1996), and dissolution of water-soluble organic substances (Facchini et al., 1999; Shulman et al., 1996). These chemical characteristics of cloud droplets lead to different heating/cooling rates. A number of variables including the chemical properties of individual cloud droplets and the solute (or whole droplet) composition are essential prerequisites for understanding cloud formation processes and heating/cooling rates.
Until recently, little was known about the chemical composition of individual microdroplets (e.g., cloud and fog droplets). This is partly due to their small size, since chemical analyses typically require a minimum sample volume much greater than that of a single drop.
Fortunately, in recent years, capillary zone electrophoresis (Tenberken and Bächmann, 1998) and replication techniques (Ma et al., 2003) have been developed to allow for the chemical analysis of a single fog or cloud droplet. However, the capillary zone electrophoresis technique of Tenberken and Bächmann was studied using only ionic species, while Ma et al.’s replication technique provided the elemental distribution of a flattened replica droplet. Therefore, both of these techniques suffer from important limitations.
Accurate identification of the spatial elemental distribution of a solid phase insoluble particle acting as CCN in a droplet is expected to be one of the most useful ways to estimate cloud microphysics (e.g., condensation, particle capture, and coalescence). Moreover, this will be a key technique for understanding the rainout mechanisms of pollutants and optical properties of clouds. In addition, information about the size distribution of cloud droplets is an important factor for estimating the precipitation efficiency of clouds and the chemical composition of rain (Kuba and Takeda, 1983).
In this study, to visualize and quantify elements micro-scale droplets (e.g., cloud and fog droplets), a combined technique for droplet fixation and an SR-XRF microprobe analysis were utilized. We report the outcome of our preliminary study and attempted to determine quantitative measures of elemental mass in individual cloud droplets.
2. EXPERIMENTAL METHODS
2. 1 Solidification Processes of Individual Micro-scale Droplets
Previous studies have clearly shown that droplet solute concentration is size-dependent (Reilly et al., 2001; Ogren and Charlson, 1992; Pandis and Seinfeld, 1990). Hence, accurate droplet sizing is critical for the proper estimation of the droplet size-dependence of elemental concentration.
Droplets, unlike solid particles, are unstable. Any contact with a solid surface (e.g., wall contact within the probe and contact with the collecting plate) disrupts the droplet, essentially destroying its structure. Also, due to droplet transformation (hemispherical shape) on the collecting plate, precise droplet sizing is difficult. Therefore, there is no adequate method of droplet collection onto a filter medium (or glass plate) for subsequent size and chemical analyses. In addition, fine mists, consisting of droplets around several tens of micrometers, are readily affected by evaporation. In addition, it is necessary that droplets remain spherical during handling and analysis processes.
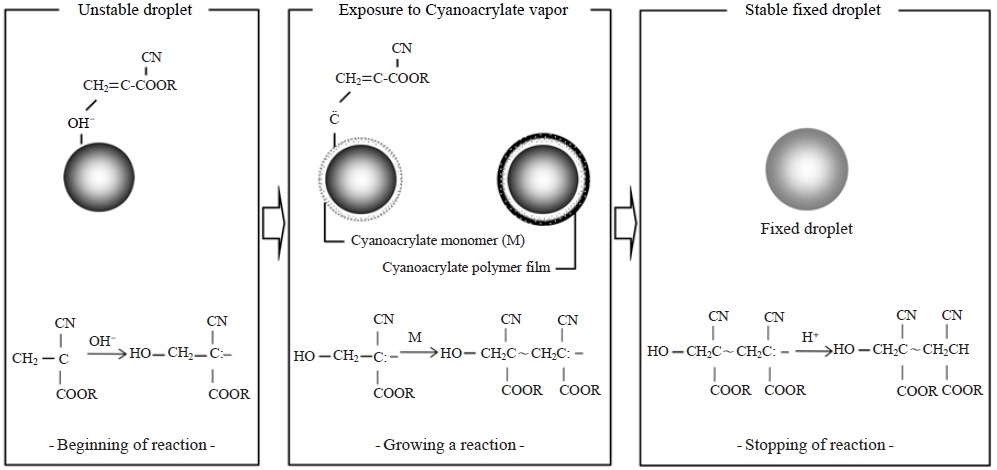
To preserve droplets in their original shapes, a fixation technique must be applied. Such a technique was first introduced by Johannsen (1965) for the replication of snow crystals and biological specimens with a resin vapor. Carter and Hasegawa (1975) and Kasahara et al. (2000) subsequently applied this fixation method to study the size distribution of tobacco smoke and to determine the chemical analysis of individual fog droplets using α-cyanoacrylate vapor, respectively.
For the fixation of liquid aerosols in the current study, α-cyanoacrylate vapor was used, and the process is schematically illustrated in Fig. 1. The fixation pathway can be divided into three stages: (1) reaction initiation due to exposure of the α-cyanoacrylate monomer vapor to the water droplet (unstable droplet), (2) reaction continuation through polymerization reactions between the α-cyanoacrylate monomers (not complete fixation), and (3) reaction completion (stable fixed droplet). More details about this fixation technique are described in a previous publication (Carter and Hasegawa, 1975).
2. 2 Experimental System in the Laboratory
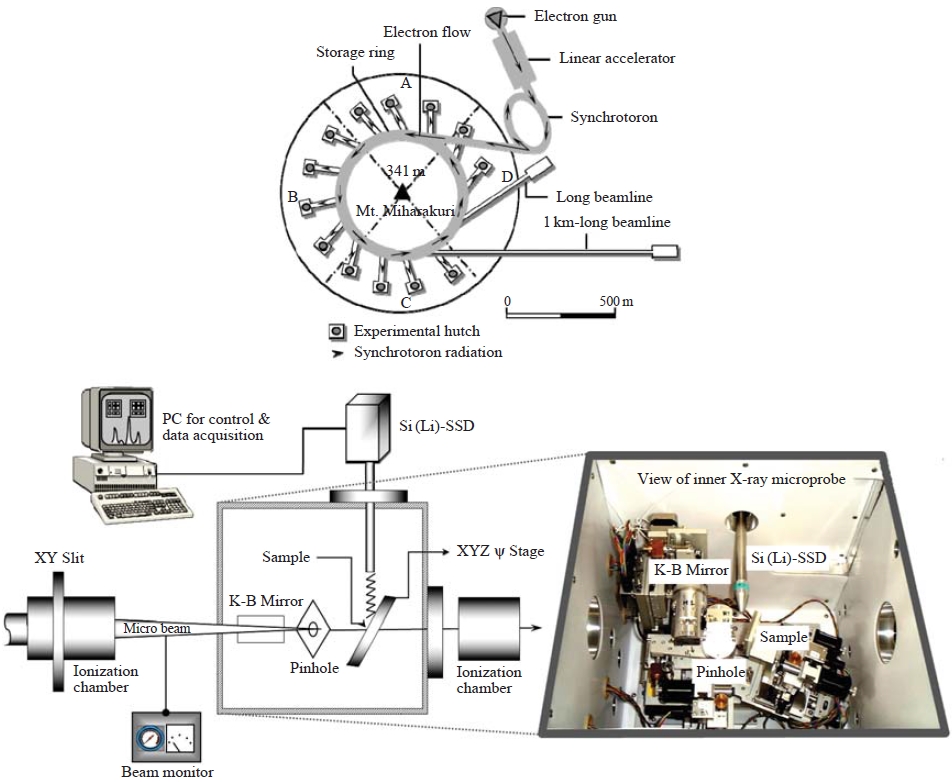
To handle individual droplets without evaporation or contamination, we designed a clean air chamber system. Every fixation process was performed in this clean air chamber filled with cooled (below 4 degrees Celsius) nitrogen gas. Fig. 2 shows a diagram of the experimental system consisting of a cool N2 gas distributor, a liquid sample dispenser, and a small-scale chamber for fixation of individual microdroplets.
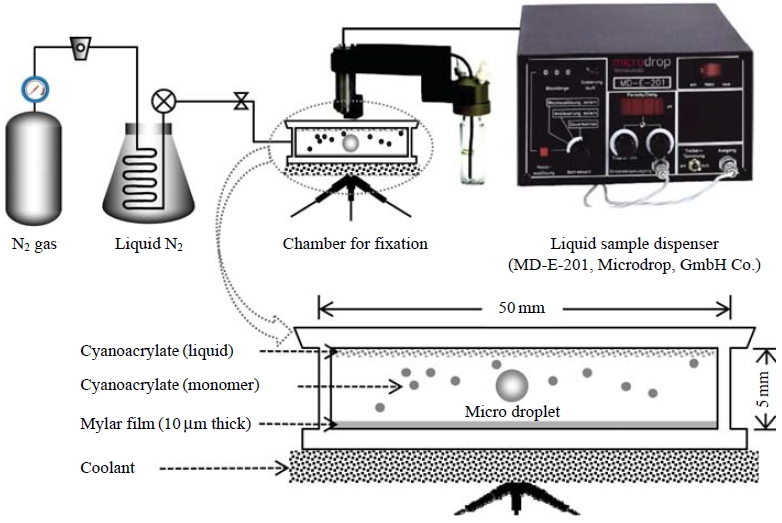
Schematic diagrams of laboratorial experimental system (top) and a small scale chamber for fixation of individual micro droplets (down).
To generate and determine a low volume liquid sample, which was adjusted to the sizes (around several tens of micrometers) of ambient droplets (e.g., cloud and fog droplets), a liquid sample dispenser (MD-E-201, Microdrop, GmbH Co.) was employed. This microdroplet dispenser was comprised of a piezoelectric transducer attached to a glass capillary, a positive displacement pump for priming and aspirating liquid into the microdispenser, a pressure controller for the liquid system, a system for washing the microdispenser between liquid transfers, and a pressure sensor to measure the liquid system pressure and to produce a corresponding electrical signal. The pressure signal was used to verify and quantify the microvolume dispensed and was used to perform automated calibration and diagnostics on the microdroplet dispenser.
A fine microdroplet was generated at the nozzle tip and was propelled from the dispenser head. In a previous study, Kasahara et al. (2003) reported that fine liquid droplets with a diameter range of a few to hundreds of μm can be fixed using the employed fixation reaction. In the present study, the size of the drop, which depends on the capillary opening, was adjusted to 50 μm. After detaching from the nozzle tip, individual microdroplets were placed onto the non-hole Nuclepore filter in the fixation chamber. These microdroplets underwent polymerization of the α-cyanoacrylate monomer evaporated from a sample of liquid cyanoacrylate placed on the ceiling of the reaction chamber.
2. 3 Chemical Analysis
To visualize and quantify the elemental compositions in individual micro-scale droplets, an X-ray microprobe system equipped at the beam line 37XU of Super Photon ring 8 GeV (SPring-8) was applied. SPring-8, the world’s largest third-generation synchrotron radiation facility, provides the most powerful synchrotron radiation currently available.
Synchrotron radiation is an electro-magnetic wave emitted from an electron traveling at almost the speed of light whose path is bent by a magnetic field. Synchrotron radiation is characterized by its useful features of brightness, high directionality, and variable polarization.
By determining the energy (wavelength) of the X-ray energy (photon) emitted by a particular element in the sample, the identity of that element can be determined. For a particular energy (wavelength) of fluorescent light emitted by an element, the number of photons per unit time (generally referred to as peak intensity or count rate) is related to the amount of that analyte in the sample. This complex but well understood interaction of X-rays with target matter allows an analyzer to yield quantitative results to a degree that is lacking in most other microscopic analytical methods. Through an application of a micro-analytical technique based on X-ray fluorescence (XRF), multiple elements were successfully analyzed at femtogram levels.
Drolets individually deposited onto the non-hole Nuclepore® polycarbonate (hydrophobic) film (25mm diameter) were placed on the XY scanning stage in a vacuum chamber (inner X-ray microprobe in Fig. 3). Sample areas (500-1,000 μm2 each time) were selected and then scanned by the microbeam. A takeoff angle of 10° was used for the measurement of X-ray fluorescence, and the intensities of the incident X-rays were monitored using an ionization chamber. The XRF elemental image of the sample was obtained via the scanning processes, and point analysis of individual droplets was subsequently performed. Fluorescence X-rays were recorded with a Si(Li) detector placed in the electron orbit plane of the storage ring. Details of the X-ray microprobe system calibration to determine quantitative measures of elemental mass in the target specimen including the effects of self-absorption are described elsewhere (Ma et al., 2010; Hayakawa et al., 2001).
3. RESULTS AND DISCUSSION
3. 1 Originally Solidified Spherical Droplets
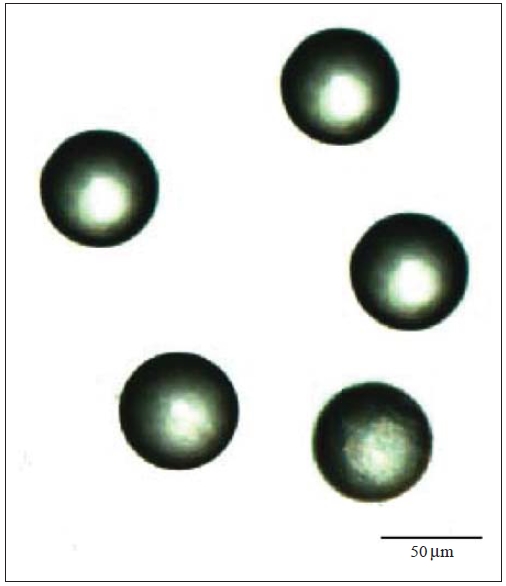
Spherical micro-scale droplets were successfully solidified onto a non-hole Nuclepore® polycarbonate filter in their original sizes and shapes (Fig. 4). When the substrate material (e.g., filter or thin film) is hydrophilic (high energy e.g., glass), water droplets tend to spread over the surface. For hydrophobic (low energy e.g., Teflon) surfaces, the water aggregates, forming a spherical cap on the substrate, positioned at a specific contact angle.
The fine, spherical micro-scale droplets which were released from the dispenser head are passively transferred onto the surface of the substrate without transformation or disruption. Preservation of droplet size and shape were demonstrated through comparison of the adjusted size (50 μm) of the microdroplet dispenser and the size (50 μm) of the digital microscopic images of the fixed droplets (Fig. 4). These results suggest that there was almost no size change after fixation. In our previous study (Kasahara et al., 2003), fixed droplets maintained their original shapes and were stable even under a 10-5 Torr vacuum condition. Consequently, this fixation technique allowed not only direct visualization but also measurement of droplet size via microscopic observation. The results were helpful for estimating the optical properties and precipitation efficiencies of clouds.
3. 2 Visualization and Relative Quantification of Elements in a Microdroplet
There are many water-soluble compounds in atmospheric aerosol, and widely variable degrees of solubility exist among them. Sulfates, sodium chloride, other water-soluble salts, and inorganic acids are common components of atmospheric aerosol and often act as CCN (Hudson and Da, 1996). Although the CCN activities of inorganic aerosol substances are well known, highly soluble gases, such as HNO3 and HCl, can also be dissolved into growing solution droplets prior to activation (Laaksonen et al., 1998; Kulmula et al., 1993).
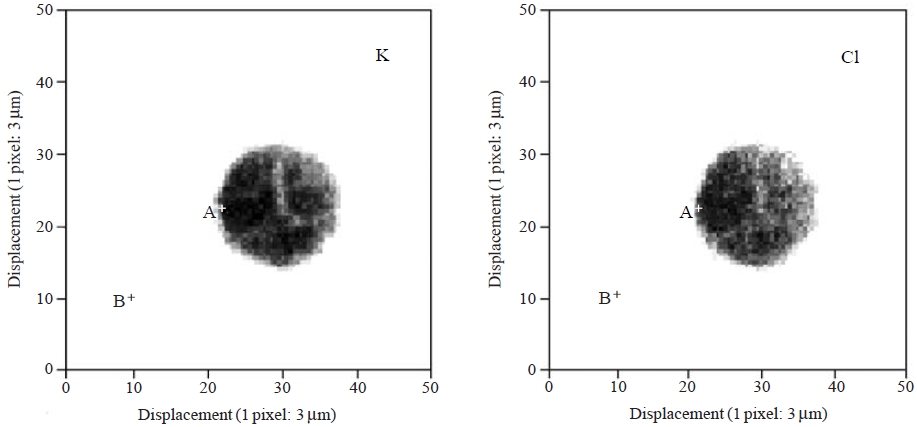
Since the micro XRF method applied in this study cannot be used to identify light-weight elements (atomic number <13), a 10-ppm KCl solution was prepared by diluting the stock KCl standard solution (MonotaRO Co., Ltd.) to generate synthetic fine liquid droplets containing inorganic water-soluble compounds. This standard solution was transformed into microscale droplets through the microdroplet dispenser and was then irradiated by an X-ray microbeam after solidification due to cyanoacrylate monomer. A 10-keV X-ray microbeam with a 5 μm diameter was scanned over an area of 150×150 μm2.
Fig. 5 illustrates the XRF elemental maps for the K and Cl contained in a microdroplet of the 10-ppm solution. A complete description is not possible here because of the lack of analytical results at this preliminary stage. Due to the use of a standard KCl solution, uniform distributions of K and Cl were observed. However, future modifications to our system (e.g., a smaller, ~1 μm beam diameter with high resolution around 1-2 μm pixel) will allow for the determination of portion-to-portion dissimilarity in the chemical states of individual microdroplets. Detailed information of elemental micro-distribution will enable us to visually estimate the following:
- 1. The compositions of particles serving as nuclei for droplet formation;
- 2. The distributions of soluble gases with different chemical components;
- 3. The water solubilities of nuclei particles;
- 4. The coalescence of droplets due to the elemental inhomogeneity of different nuclei;
- 5. The relationship between the chemical components and the growth rates of individual droplets.
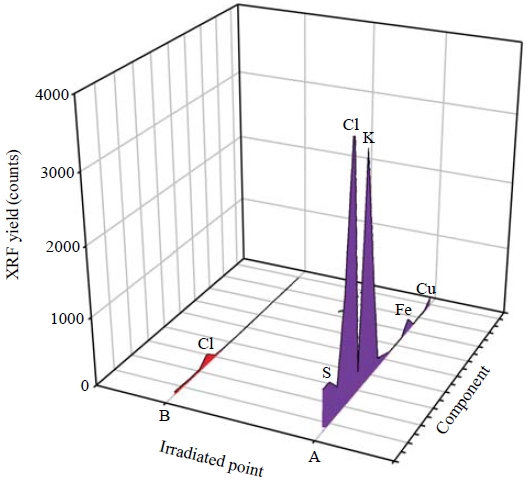
Multi-elemental peaks corresponding to X-ray energy were successfully resolved. Fig. 6 displays the XRF spectra for two points marked on the XRF elemental maps (Fig. 5). Two different positions (i.e., on a fixed droplet and on blank filter medium) were subjected to XRF analysis. The low XRF counts for Cu and Fe were probably caused by the sample holder of the XRF microprobe analytical system. Peaks for S and Cl were presumably derived from impurities in the α-cyanoacrylate and the Nuclepore® polycarbonate film.
To quantify elements in a microdroplet, the selfabsorption of X-rays could be studied. Self-absorption of primary X-rays and emitted X-ray fluorescence may be caused by the relative thickness of a fixed droplet (from several tens to several hundreds of μm). However, Liang et al. (2008) successfully visualized X-ray fluorescence images of K, Ca and Fe at different depths (200, 400, and 500 μm) in a kernel of rice grain. The samples in the present study were quite thin (50 μm) compared to a rice grain, and homogenous elemental distribution were less affected by the selfabsorption of X-rays.
3. 3 Extended Efforts to Determine Quantitative Measurements of Elemental Mass in Individual Droplets
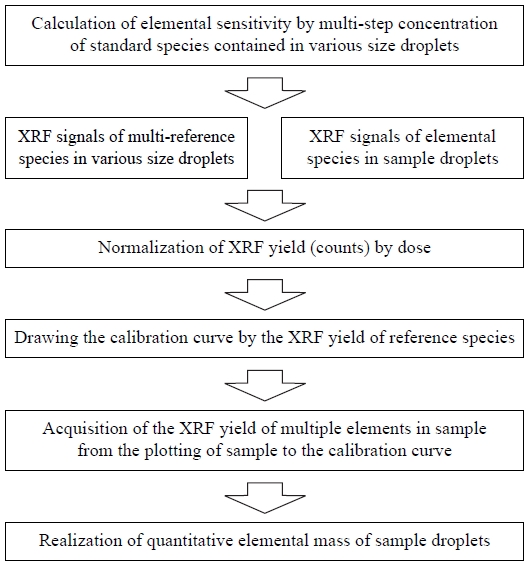
The procedure of quantitative measurement for elemental mass in individual droplets is summarized in Fig. 7. Reference samples with various droplet sizes and multiple elemental compositions should be prepared as the first step of this quantitative measurement. Then the elemental sensitivities of the multi-step concentrations of standard species contained in various droplet sizes must be accurately determined. To this end, the XRF signals of the reference samples and sample droplets were measured for several hundred seconds of data acquisition time, and XRF yield (i.e., counts) was normalized according to dose. The XRF yields of multiple elements in the sample were then calculated by plotting the sample results on the multielement calibration curve. Finally, the elemental mass quantities in the sample droplets can be determined.
4. CONCLUSIONS
Quantitative analysis of elemental compositions in individual droplets can provide new and interesting information on the impacts of aerosol on global cloud formation, a phenomenon known as the “aerosol indirect climatic effect”. Also, the liquid droplet phase is important for proper performance of the heterogeneous multi-phase processes of the troposphere. Knowledge of the chemical compositions of CCN particles and of the gaseous materials dissolved into droplets is essential. Although it is possible to obtain more detailed information from the above experiments, there were some difficulties in the sampling and analytical processes of individual droplets due to difficulty in their handling, evaporation, and contamination.
The objective of this study was to quantify the elemental compositions in individual microdroplets using solidification and synchrotron radiation techniques. Although a thorough interpretation is not possible due to limited data, it is possible to say that the combination of the fixation method and the SR-XRF technique is a suitable analytical method to determine elements in individual cloud droplets. To produce 3D visualization and quantification of the elemental natures of individual droplets, especially carbon content, further experiments are needed.
Acknowledgments
This study was supported in part by funds from the Grant-in-Aid for Scientific Research on Priority Areas under Grant Nos. 14048212 and 14048213 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This study was also partially supported by aid from the program “Establishment of COE on Sustainable-Energy System.” The synchrotron radiation experiments were performed at the SPring-8 facility with approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2002B0395-NOS-np, 2002A4029-LM-np).
The authors wish to express thanks to all the members of SPring-8, BL-37XU.
REFERENCES
- Albrecht, B.A., (1989), Aerosols, cloud microphysics, and fractional cloudiness, Science, 461, p1227-1230.
-
Carter, W.L., Hasegawa, I., (1975), Fixation of tobacco smoke aerosols for size distribution studies, Journal of Colloid Interface Science, 53, p134-141.
[https://doi.org/10.1016/0021-9797(75)90044-2]

-
Facchini, M.C., Mircea, M., Fuzzi, S., Charlson, R.J., (1999), Cloud albedo enhancement by surface-active organic solutes in growing droplets, Nature, 401, p257-259.
[https://doi.org/10.1038/45758]

-
Hayakawa, S., Ikuta, N., Suzuki, M., Wakatsuki, M., Hirokawa, T., (2001), Generation of an X-ray microbeam for spectromicroscopy at SPring-8 BL39XU, Journal of Synchrotron Radiation, 8, p328-330.
[https://doi.org/10.1107/s0909049500018446]

-
Hudson, J., Da, X., (1996), Volatility and size of cloud condensation nuclei, Journal of Geophysical Research, 101, p4435-4442.
[https://doi.org/10.1029/95jd00192]

- Intergovernmental Panel on Climate Change (IPCC), The Fourth Assessment Report (AR4), (2007), http://en.wikipedia.org/wiki/IPCC_Fourth_Assessment_Report.
- Johannsen, R.I.S., (1965), Resin vapor replication technique for snow crystals and biological specimens, Nature, 205, p1204-1205.
-
Jones, A., Roberts, D.L., Woodage, M.J., (2001), Indirect sulphate aerosol forcing in a climatic model with an interactive sulphur cycle, Journal of Geophysical Research, 106, p20293-30310.
[https://doi.org/10.1029/2000jd000089]

- Kasahara, M., Akashi, S., Ma, C.J., Tohno, S., Ohnishi, Y., (2000), Physicochemical characteristics of individual fog droplets and raindrops, Environmental Conservation Engineering, 29, p822-827, (in Japanese).
- Kasahara, M., Akashi, S., Ma, C.-J., Tohno, S., (2003), Fixation and chemical analysis of individual fog droplet and raindrop, Atmospheric Research, 65, p251-259.
-
Kuba, N., Takeda, T., (1983), Numerical study of the effect of CCN on the size distribution of cloud droplets, Journal of the Meteorological Society of Japan, 61, p375-387.
[https://doi.org/10.2151/jmsj1965.61.3_375]

- Kulmula, M., Laaksonen, A., Korhonen, P., Vesala, T., Ahonen, T., (1993), The effect of atmospheric nitric acid vapor on cloud condensation nucleus activation, Journal of Geophysical Research, 98, p22949-22958.
-
Laaksonen, A., Korhonen, P., Kulmala, M., Charlson, R.J., (1998), Modification of the Kohler equation to include soluble trace gases and slightly soluble substances, Journal of Atmospheric Science, 55, p853-862.
[https://doi.org/10.1175/1520-0469(1998)055<0853:motkhe>2.0.co;2]

- Liang, J., Li, Z., Tsuji, K., Nakano, K., Nout, M.J.R., Hamer, R.J., (2008), Milling characteristics and distribution of phytic acid and zinc in rice kernels, Journal of Cereal Science, 48, p83-91.
-
Lohmann, U., Feichter, J., (2005), Global indirect aerosol effects: a review, Atmospheric Chemistry and Physics, 5, p715-737.
[https://doi.org/10.5194/acp-5-715-2005]

-
Ma, C.-J., Kasahara, M., Tohno, S., Sakai, T., (2003), A replication technique for the collection of individual fog droplets and their chemical analysis using micro-PIXE, Atmospheric Environment, 37, p4679-4686.
[https://doi.org/10.1016/j.atmosenv.2003.07.003]

-
Ma, C.J., Kim, J.H., Kim, K.H., Tohno, S., Kasahara, M., (2010), Specification of chemical properties of feed coal and bottom ash collected at a coal-fired power plant, Asian Journal of Atmospheric Environment, 4(2), p80-88.
[https://doi.org/10.5572/ajae.2010.4.2.080]

-
Menon, S., Del Genio, A.D., Koch, D., Tselioudis, G., (2002), GCM simulations of the aerosol indirect effect: Sensitivity to cloud parameterization and aerosol burden, Journal of Atmospheric Science, 59, p692-713.
[https://doi.org/10.1175/1520-0469(2002)059<0692:gsotai>2.0.co;2]

-
Ogren, J.A., Charlson, R.J., (1992), Implications for models and measurements of chemical inhomogenities among cloud droplets, Tellus, 44B, p208-225.
[https://doi.org/10.3402/tellusb.v44i3.15443]

-
Pandis, S.N., Seinfeld, J.H., (1990), Chemical composition differences in fog and cloud droplets of different sizes, Atmospheric Environment, 24A, p1957-1969.
[https://doi.org/10.1016/0960-1686(90)90529-v]

-
Reilly, J.E., Rattigan, O.V., Moore, K.F., Judd, C., Sherman, D.E., Dutkiewicz, V.A., Kreidenweis, S.M., Husain, L., Collett, J.L. Jr., (2001), Drop size-dependent S(IV) oxidation in chemically heterogeneous radiation fogs, Atmospheric Environment, 35, p5717-5728.
[https://doi.org/10.1016/s1352-2310(01)00373-9]

-
Shulman, M., Jacobson, M., Charlson, R., Synovec, R., Young, T., (1996), Dissolution behavior and surface tension effects of organic compounds in nucleating cloud droplets, Geophysical Research Letter, 23, p277-280.
[https://doi.org/10.1029/95gl03810]

-
Tenberken, B., Bächmann, K., (1998), Sampling and analysis of single cloud and fog drops, Atmospheric Environment, 32, p1757-1763.
[https://doi.org/10.1016/s1352-2310(97)00461-5]

-
Twomey, S., (1977), The influence of pollution on the shortwave albedo of clouds, Journal of Atmospheric Science, 34, p1149-1152.
[https://doi.org/10.1175/1520-0469(1977)034<1149:tiopot>2.0.co;2]