
Experimental Studies on the Effects of Ozone on Growth and Photosynthetic Activity of Japanese Forest Tree Species
Abstract
Ozone (O3) is a main component of photochemical oxidants, and a phytotoxic anthropogenic air pollutant. In North America and Europe, the current concentration of O3 has been shown to have significant adverse effects on vegetation. In this review, we summarize the experimental studies on the effects of O3 on the growth and photosynthetic activity of Japanese forest tree species to understand the present knowledge and provide sound basis for future research toward the assessment of O3 impacts on Japanese forest ecosystem. Since the 1990s, several Japanese researchers have conducted the experimental studies on the effects of ambient levels of O3 on growth and physiological functions such as net photosynthesis of Japanese forest tree species. Although the sensitivity to O3 of whole-plant growth is quite different among the species, it was suggested that the current ambient levels of O3 in Japan are high enough to adversely affect growth and photosynthetic activity of Japanese forest tree species classified into high O3 sensitivity group such as Japanese beech. The N load to soil has been shown to reduce the sensitivity to O3 of Japanese larch and increase that of Japanese beech. To establish the critical level of O3 for protecting Japanese forest tree species, therefore, it is necessary to take into account the N deposition from the atmosphere. There is little information on the combined effects of O3 and other environmental factors such as elevated CO2 and drought on growth and physiological functions of Japanese forest tree species. Therefore, it is necessary to promote the experimental study and accumulate the information on the combined effects of O3 and any other abiotic environmental factors on Japanese forest tree species.
Keywords:
Ozone, Japanese forest tree species, Growth, Photosynthetic activity, Critical level1. INTRODUCTION
Ozone (O3) is a main component of photochemical oxidants and produced by photochemical reaction of volatile organic compounds (VOCs) and nitrogen oxide (NOx) (ADORC, 2006; EPA, 2006a). Ozone adversely affects not only human health but also vegetation (ADORC, 2006; EPA, 2006a). The emissions of precursor for O3 from Asian countries have rapidly increased since the 1970s and surpassed the emissions from North America and Europe in the mid-1990s (Ohara et al., 2007; Akimoto, 2003). This situation is expected to continue for at least next couple decades (Klimont et al., 2001). In the near future, therefore, the concentration of ground-level O3 is expected to increase especially in Asian countries including Japan (Yamaji et al., 2008; Dentener et al., 2006; Derwent et al., 2002; Emberson et al., 2001).
Since the ambient levels of O3 in the USA and Europe negatively affect growth and physiological functions such as photosynthesis of forest tree species, this gas is considered as one of the important factors relating to forest decline and tree dieback in the relevant regions (Bytnerowicz et al., 2004; Chappelka and Samuelson, 1998; Skärby et al., 1998; Sandermann et al., 1997). In Japan, relatively high concentrations of O3 above 100 nL L-1 (ppb) have been frequently observed from spring to autumn in several mountainous areas (Kohno et al., 2007; Takeda and Aihara, 2007; Aihara et al., 2004; Maruta et al., 1999). Based on the results of the experimental studies and field surveys, it has been suggested that O3 is an important environmental stress relating to the forest decline in Japan (Kume et al., 2009; Suto et al., 2008; Takeda and Aihara, 2007; Yamaguchi et al., 2007b; Kohno et al., 2005; Aihara et al., 2004; Yonekura et al., 2001a, b; Maruta et al., 1999).
In Japan, pioneer studies on the effects of O3 on woody plants were published in the 1970s. Nouchi et al. (1973a, b) and Matsushima et al. (1977) mainly focused on O3-induced visible injury and changes in the ultrastructural characteristics of leaves of several tree species. Kuno (1980, 1979), Furukawa et al. (1983), Fujinuma et al. (1987) and Furukawa (1991) reported the effects of O3 on growth or physiological functions such as net photosynthesis of street trees. Matsumoto et al. (1992) reported the effects of O3 at remarkably high concentrations on needle gas exchange rates of Japanese cedar (Cryptomeria japonica). Since the 1990s, the experimental studies on the effects of ambient levels of O3 on growth, phenological characteristics and physiological functions such as net photosynthesis of Japanese forest tree species have started. To provide sound basis for future research toward the assessment of O3 impacts on Japanese forest ecosystems, in this review, we summarize the experimental studies hitherto reported on the effects of O3 on growth and photosynthetic activity of Japanese forest tree species.
2. EFFECTS OF O3 ON GROWTH OF JAPANESE FOREST TREE SPECIES
Table 1 indicates the summary of experimental studies on the effects of O3 on Japanese forest tree species. Miwa et al. (1993) reported that the exposure of Japanese cedar seedlings to relatively high O3 concentration (300 ppb) did not induce significant reduction in the whole-plant dry mass, but induced significant increase in the ratio of the above-ground dry mass to root dry mass (Top/Root ratio). Izuta et al. (1996) reported that the whole-plant dry mass and root dry mass of Japanese beech (Fagus crenata) seedlings were reduced by the exposure to ambient levels of O3 (75 and 150 ppb). Yonekura et al. (2001a, b) also reported that the exposure to ambient level of O3 (60 ppb) reduced dry masses of root, leaf and stem, the whole-plant dry mass and annual ring width of Japanese beech seedlings. Nakaji and Izuta (2001) and Nakaji et al. (2004) reported that dry masses of needles and fine roots and the whole-plant dry mass of Japanese red pine (Pinus densiflora) seedlings were reduced by the exposure to ambient level of O3 (60 ppb). Aforementioned studies were conducted using the steady-state O3 exposure system. Because there are seasonal and diurnal variations in tropospheric O3 concentration (Khiem et al., 2010; EPA, 2006a; Yamaji et al., 2006), O3 exposure system with the variations in the atmospheric concentration of O3 observed in the fields needs to be used for the evaluation of the realistic effects of O3 on forest tree species. Matsumura et al. (1996) and Matsumura et al. (1998) conducted experimental studies on the effects of O3 on the growth of several Japanese forest tree species using an O3 exposure system with seasonal and diurnal variations in the atmospheric concentration of O3. In the study of Matsumura et al. (1996), the seedlings of Japanese cedar, Japanese cypress (Chamaecyparis obtusa) and Japanese zelkova (Zelkova serrata) were exposed to O3 at 0.4, 1.0, 2.0 and 3.0 times the ambient concentration (12-h (6:00-18:00) average concentration of O3: 16, 39, 74 and 114 ppb, respectively). The whole-plant dry mass of Japanese zelkova exposed to 2.0 and 3.0 times the ambient concentration of O3 and that of Japanese cedar exposed to 3.0 times the ambient concentration of O3 were significantly lower than those exposed to 0.4 times the ambient concentration of O3, while there was no significant effect of O3 on the whole-plant dry mass of Japanese cypress. In the study of Matsumura et al. (1998), the seedlings of Japanese cedar, Nikko fir (Abies homolepis), Japanese white birch (Betula platyphylla) and Japanese zelkova were exposed to O3 at 0.4, 1.0, 2.0 and 3.0 times the ambient concentration (12-h (6:00-18:00) average concentration of O3: 18, 37, 67 and 98 ppb, respectively). The whole-plant dry mass of Japanese cedar, Japanese white birch and Japanese zelkova were decreased linearly with increasing the concentration of O3, while that of Nikko fir was not. These results indicate that the sensitivity to O3 of the whole-plant growth is quite different among the Japanese forest tree species.
Aforementioned experimental studies were conducted within one growing season. However, there are several reports concerning the carry-over effects of O3 on perennial plants. For example, the exposure to O3 during one growing season changes phenological characteristics such as delay in the timing of bud break, and reduces leaf number per bud and growth in the following growing season (Yonekura et al., 2004; Oksanen and Saleem, 1999; Andersen et al., 1997; Pearson and Mansfield, 1994). Therefore, multi-year experiments are crucial to assessing the degree of adverse effects of O3 on the growth of forest trees (Ashmore, 1993). Matsumura (2001) conducted the multi-year experiments. Young trees of 14 species were exposed to charcoal-filtered air (CF) or non-filtered air (NF) for three growing seasons at two different sites in Kanto districts of Japan (Chiba Prefecture and Gunma Prefecture; 12-h (6:00-18:00) seasonal mean concentration of O3 (from April to September) during the experimental period in CF treatments: 8 and 12 ppb, respectively; those in NF treatments: 26 and 37 ppb, respectively). The ambient levels of O3 reduced the whole-plant dry mass of Japanese red pine, Japanese larch (Larix kaempferi), Veitch’s silver fir (Abies veitchii), Japanese white birch, Japanese beech and Japanese zelkova at the both sites. Kohno et al. (2005) summarized several results of experimental studies conducted for multiple growing seasons on the effects of O3 on forest tree species (e.g. Matsumura, 2001; Matsumura and Kohno, 1999). The sensitivity of each tree species to O3 was classified into 3 groups (high, moderate and low) based on the response of the whole-plant dry mass growth to O3 (Table 2). For example, Japanese larch and Japanese beech have been classified into high O3 sensitivity group; Japanese white birch and Nikko fir have been classified into moderate O3 sensitivity group; Japanese cedar and Japanese cypress have been classified into low O3 sensitivity group. Recently, Takeda and Aihara (2007) showed that O3 negatively affects growth and photosynthetic parameters of Japanese beech grown under field condition at Tanzawa Mountains where the decline of Japanese beech forest has been reported. Kume et al. (2009) suggested the possibility that recent increase in the atmospheric concentration of O3 is an important factor of Japanese beech decline at Mt. Tateyama based on the results of their field survey. These results and those obtained from the experimental studies clearly indicate that current ambient levels of O3 in Japan are high enough to adversely affect growth of Japanese forest tree species especially in those classified into high O3 sensitivity group such as Japanese beech.
3. EFFECTS OF O3 ON THE PHOTOSYNTHETIC ACTIVITY AND OTHER RELATED FUNCTIONS OF JAPANESE FOREST TREE SPECIES
Izuta et al. (1996) and Matsumura et al. (1996) reported that relative growth rates (RGRs) of Japanese beech, Japanese cedar and Japanese zelkova were reduced by the exposure to O3. In their studies, the O3-induced reductions in net assimilation rate (NAR) and net photosynthetic rate of the leaves or needles were reported. These results indicate that the O3-induced reduction in the growth was mainly due to that in the net photosynthetic rate of the leaves or needles. It was also reported that the exposure to O3 reduced the net photosynthetic rate of the leaves or needles of Japanese white birch, Japanese red pine, Japanese larch, Q. serrata and C. sieboldii (Watanabe et al., 2008, 2007, 2006; Nakaji et al., 2004; Nakaji and Izuta, 2001; Matsumura et al., 1998). When net photosynthetic rate was reduced by the exposure to O3, simultaneous reductions in the carboxylation efficiency (CE), CO2-saturated net photosynthetic rate (Amax) and/or maximum quantum yield of photosystem (PS) II (Fv/Fm), and increase in the stomatal diffusive conductance to water vapor (gs) in the leaves or needles of Japanese forest tree species were also observed (Yamaguchi et al., 2007a; Watanabe et al., 2005; Nakaji and Izuta, 2001; Yonekura et al., 2001a, b; Matsumura et al., 1998, 1996; Izuta et al., 1996). It was documented that the exposure to O3 reduced the concentration and activity of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and chlorophyll concentration in the leaves or needles of Japanese forest tree species (Watanabe et al., 2007, 2005; Yamaguchi et al., 2007a, b; Nakaji et al., 2004; Nakaji and Izuta, 2001; Yonekura et al., 2001a; Izuta et al., 1996; Miwa et al., 1993). Yonekura et al. (2001b) reported that the O3-induced reduction in net photosynthetic rate was firstly due to the reduction in the quantity and/or activity of Rubisco in the leaves of Japanese beech. Therefore, there is a possibility that the exposure to O3 firstly reduces the capacity of carbon fixation in the chloroplasts resulting in the reduction in net photosynthetic rate of the leaves or needles of Japanese forest tree species.
Proteins such as Rubisco in the leaves or needles represent the predominant N fraction (Feller, 2004; Spreitzer and Salvucci, 2002). Ozone exposure has been shown to reduce the concentration of total soluble protein (TSP) in the leaves of Q. serrata and Japanese beech(Watanabe et al., 2007; Yamaguchi et al., 2007a, b). Watanabe et al. (2007) and Yamaguchi et al. (2007b) reported that the exposure to O3 reduced photosynthetic nitrogen use efficiency (PNUE) in the leaves of Q. serrata and Japanese beech. In the case of Japanese beech, the exposure to O3 did not significantly affect N concentration in the leaves, suggesting that O3 induces alterations in foliar N metabolism and also a reduction in the availability of N for photosynthesis in the leaves (Yamaguchi et al., 2007b). There is limited information on the effects of O3 on N metabolism in the leaves or needles of Japanese forest tree species (Yamaguchi et al., 2010, 2007a; Nakaji et al., 2004). Nakaji et al. (2004) reported that the exposure to O3 did not significantly affect the activities of nitrate reductase (NR) and nitrite reductase (NiR) and concentrations of inorganic N compounds (NO3-, NO2- and NH4+) and free amino acid in the needles of Japanese red pine. On the other hand, Yamaguchi et al. (2010, 2007a) reported the O3-induced inhibition of resorption of N from the leaves in autumn, reductions in the NR activity and the ratio of TSP concentration to N concentration and increase in the concentration of acidic amino acid in the leaves of Japanese beech. At the present time, it is unclear how O3 affects N metabolism in the leaves of Japanese forest tree species. To clarify the mechanisms underlying the detrimental effects of O3 on Japanese forest tree species, therefore, further research concerning the effects of O3 on physiological functions such as foliar N metabolism is required.
4. COMBINED EFFECTS OF O3 AND OTHER ABIOTIC ENVIRONMENTAL FACTORS ON JAPANESE FOREST TREE SPECIES
Izuta (2002, 1998) and Izuta et al. (2001) reviewed experimental studies on the combined effects of O3 and simulated acid rain on Japanese forest tree species. Recently, it was pointed out that the interactive effects of O3, N deposition, elevated carbon dioxide (CO2) and climate change such as drought stress must be key issues to predict forest future in the changing environment (Paoletti et al., 2010). In this section, we focused on the combined effects of O3 and N load to soil, elevated CO2 or drought on growth, photosynthetic activity and other related functions of Japanese forest tree species.
4. 1 Nitrogen Load to Soil
Atmospheric deposition of N to terrestrial ecosystems has been increasing with elevated anthropogenic emissions of N since the industrial revolution (Richter et al., 2005; Galloway et al., 2004, 2003; IPCC, 2001). Because N is a limiting nutrient for plant growth in terrestrial ecosystems (Vitousek and Howarth, 1991), an increase in N input to forest ecosystems generally stimulates tree growth. However, many researchers suggested that excessive deposition of N such as nitrate and ammonium from the atmosphere to forest ecosystems might induce soil acidification, modify tree nutrient status and increase the sensitivity of trees to other environmental stresses such as gaseous air pollutants (Aber et al., 1989; Schulze, 1989; Nihlgård, 1985).
Based on the monitoring data and estimations of O3 concentration and atmospheric N deposition in East Asia (Network Center for EANET, 2011; Yamaji et al., 2006; Kohno et al., 2005), there is the possibility that forest tree species are adversely affected not only by O3, but also by excessive N deposition in East Asian countries including Japan. In the experimental studies of Watanabe et al. (2008, 2007, 2006) and Yamaguchi et al. (2010, 2007b), seedlings of Q. serrata, Japanese beech, C. sieboldii, Japanese red pine, Japanese larch and Japanese cedar were grown in potted soil supplied with N as NH4NO3 solution at 0, 20 and 50 kg ha-1 year-1 and simultaneously exposed to charcoal-filtered air or O3 at 1.0, 1.5 and 2.0 times the ambient concentration for two growing seasons (24-h seasonal mean concentration of O3 (from April to September) during the experimental period: 12, 43, 63 and 84 ppb, respectively). Watanabe et al. (2008, 2007, 2006) reported the additive effects of O3 and N load on growth of the seedlings of Q. serrata, C. sieboldii, Japanese red pine and Japanese cedar. On the other hand, significant interactive effects of O3 and N load on growth were detected in Japanese larch and Japanese beech (Yamaguchi et al., 2007b; Watanabe et al., 2006). The relationships between relative whole-plant dry mass increment of Japanese larch or Japanese beech and daylight AOT40 of O3 (accumulated exposure over a threshold of 40 ppb during daylight hours (global radiation>50W m-2), see Fig. 1) were shown in Fig. 2. Daylight AOT40 is the sum of the difference between the hourly mean O3 concentration and 40 ppb for all daylight hours (shaded area in Fig. 1) within a specified time period (from April to September in this case). The calculation of the relationships was based on the method of Karlsson et al. (2004). The coefficient of determination (R2) obtained from linear regression analysis and the slope of regression line in each N treatment are indicated in Fig. 2. The absolute value of the slope of regression line indicates the sensitivity to O3 of whole-plant dry mass growth of the seedlings. While the N load to soil reduced the sensitivity to O3 of whole-plant dry mass growth of Japanese larch (Watanabe et al., 2006), it increased that of Japanese beech (Yamaguchi et al., 2007b). These results indicate that the combined effect of O3 and N load on growth is quite different among the Japanese forest tree species.
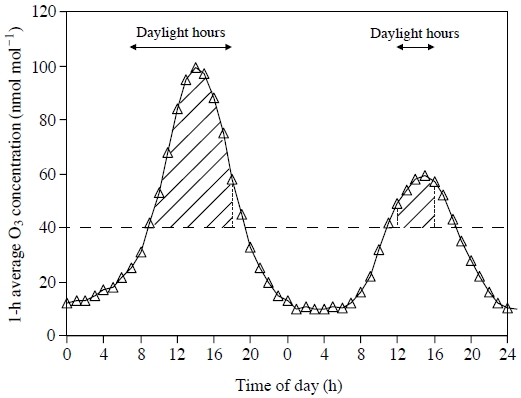
Conceptual diagram for the calculation of daylight AOT40 of O3 (accumulated exposure over a threshold of 40 ppb, nmol mol-1 h or μmol mol-1 h). Shaded area contributes to daylight AOT40. Daylight hour: global radiation >50W m-2.
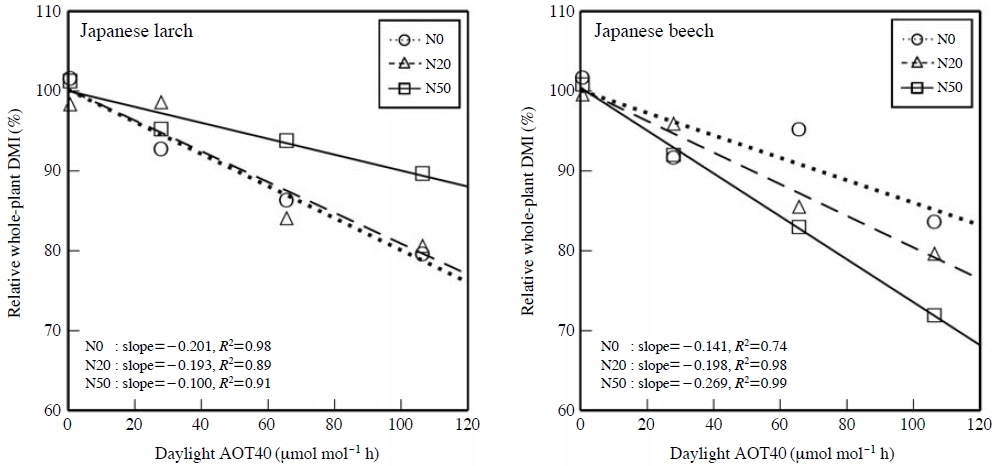
The relationships between relative whole-plant dry mass increment (DMI) of Japanese larch and Japanese beech seedlings per one growing season and daylight AOT40 of O3. The seedlings were grown in the soil supplied with N as NH4NO3 solution at 0 (N0), 20 (N20) or 50 kg ha-1 year-1 (N50) and simultaneously exposed to charcoal-filtered air or O3 at 1.0, 1.5 and 2.0 times ambient concentration. Data source: Watanabe et al. (2006) and Yamaguchi et al. (2007a, b).
Nakaji and Izuta (2001), Nakaji et al. (2004) and Watanabe et al. (2006) reported that the N load to soil did not change the degree of O3-induced reduction in net photosynthetic rate in the needles of Japanese red pine and Japanese larch. In contrast, the degrees of O3-induced reduction in net photosynthetic rate of Q. serrata, Japanese beech and C. sieboldi became high with increasing the amount of N load to soil(Watanabe et al., 2008, 2006; Yamaguchi et al., 2007b). To clarify the mechanisms underlying the combined effects of O3 and N load on net photosynthesis of Japanese forest tree species, Yamaguchi et al. (2010, 2007b) investigated the effects of O3 and N load on the concentration and activity of Rubisco, enzyme activity of N metabolism and concentrations of amino acid and soluble protein in the leaves of Japanese beech seedlings. In their studies, the seedlings of Japanese beech were grown in the soil supplied with N as NH4NO3 solution at 0, 20 or 50 kg ha-1 year-1 and simultaneously exposed to charcoal-filtered air (CF) or O3 at 1.0, 1.5 and 2.0 times ambient concentration. The exposure to O3 significantly reduced the concentration and activity of Rubisco in the leaves of the seedlings grown in relatively high N load treatment, but not in relatively low N load treatment (Fig. 3a). This result indicates that the interactive effect of O3 and N load on net photosynthetic rate is mainly attributed to the difference in the degrees of O3-induced reduction in the amount of Rubisco among the N treatments (Yamaguchi et al., 2007b). Furthermore, the exposure to O3 reduced the concentration of TSP and the ratio of TSP concentration to leaf N concentration in relatively high N load treatment, but not in relatively low N load treatment (Fig. 3b and c). Therefore, Yamaguchi et al. (2010) concluded that the exposure to O3 reduced the allocation of N to soluble protein in the leaves of Japanese beech seedlings grown under relatively high N load, but did not in the leaves of the seedlings grown under a relatively low N load.
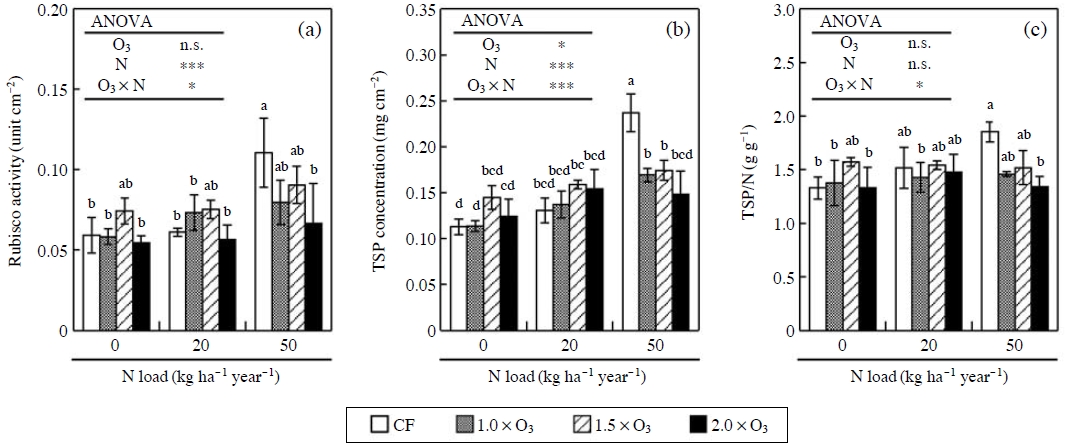
Effects of O3 and N load on activity of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), concentration of total soluble protein (TSP) and ratio of TSP to leaf N content per unit leaf area (TSP/N) in the leaves of Japanese beech. The standard deviation is given by vertical bar. Two-way ANOVA: *p<0.05, ***p<0.001, n.s.=not significant. Different letters above the bar indicate significant difference among the 12 treatments (Tukey’s HSD test, p<0.05). Data source: Yamaguchi et al. (2010, 2007b).
4. 2 Elevated CO2 and Drought
Elevated CO2 and drought are well known to affect the sensitivity of forest tree species to O3 (e.g. EPA, 2006b). Unfortunately, there is little information on the combined effects of O3 and elevated CO2 or soil water stress on Japanese forest tree species (Watanabe et al., 2010, 2005; Matsumura et al., 2005; Yonekura et al., 2001a, b).
Matsumura et al. (2005) reported that the effect of elevated CO2 on O3-induced reduction in growth was counteractive in Japanese white birch seedlings, while not in the Japanese mountain birch (Betula ermanii), Japanese beech, Japanese red pine and Japanese cedar seedlings. This result indicates that the combined effect of O3 and elevated CO2 is different among Japanese forest tree species. On the other hand, Watanabe et al. (2010) reported that the simultaneous exposure to O3 and elevated CO2 induced marked growth stimulation of Japanese beech seedlings as compared with those exposed to elevated CO2. Yonekura et al. (2001a, b) reported the additive effects of O3 and soil water stress on the growth of Japanese beech seedlings. On the other hand, Watanabe et al. (2005) reported that chronic soil water stress counteracted the negative effects of O3 on net photosynthesis of the leaves of Japanese beech seedlings. Combined effects of O3 and other environmental factors such as elevated CO2 and drought on growth and physiological functions of Japanese forest tree species are still poorly understood. Therefore, it is necessary to promote the experimental study and accumulate the information on the combined effects of O3 and any other abiotic environmental factors on growth, physiological functions and nutrient status of Japanese forest tree species.
5. CRITICAL LEVEL OF O3 FOR PROTECTING JAPANESE FOREST TREE SPECIES
The ambient levels of O3 in Japan have been shown to adversely affect growth and photosynthetic activity of Japanese forest tree species especially in those classified into high O3 sensitivity group as mentioned above. In Europe, the concept of critical level has been developed to prevent long-term injury and damage of air pollutants to the receptors such as plants (Mills et al., 2010). The critical levels for vegetation are defined as the concentration, cumulative exposure or cumulative stomatal flux of atmospheric pollutants above which direct adverse effects on sensitive vegetation may occur according to present knowledge (Mills et al., 2010). At the present time, to define the concentration-based critical levels for O3, AOT40 has been adopted for use within the United Nations Economic Commission for Europe (UNECE) Convention of Long-Range Trans-boundary Air Pollution (CLRTAP) and the European Union (Mills et al., 2010; Ashmore et al., 2004). As a result of much efforts directing to establishing the critical level of O3, critical level for forest trees has been defined as 5 μmol mol-1 h (ppm h) of daylight AOT40 accumulated over a six-month growing season (from April to September) associated with a 5% growth reduction per one growing season for sensitive deciduous tree species native to Europe such as European beech (Fagus sylvatica) and European birch (Betula pendula) (Mills et al., 2010; Karlsson et al., 2004). Because the vegetation and climatic condition in Japan is quite different from that in Europe, critical level of O3 for forest tree species in Europe is not directly applicable to that in Japan (Kohno et al., 2005). Kohno et al. (2005) proposed that provisional critical level of O3 for Japanese forest tree species classified into the high O3 sensitivity group such as Japanese larch and Japanese beech is 8-15 ppm h of daylight AOT40 accumulated over one growing season (from April to September) associated with a 10% reduction in the increment of the whole-plant dry mass per one growing season (Table 2). However, N deposition from the atmosphere should be taken into account to evaluate the critical level of O3 for protecting Japanese forest tree species, because the sensitivities of Japanese larch and Japanese beech to O3 are influenced by the amount of N load to soil (Yamaguchi et al., 2007b; Watanabe et al., 2006). Furthermore, as indicated by Matsumura et al. (2005) and Watanabe et al. (2010), it is necessary to take into account the environmental factors such as atmospheric CO2 concentration to evaluate the critical level of O3. To establish the critical level of O3 for protecting Japanese forest tree species, therefore, further research concerning the combined effects of O3 and other abiotic environmental factors on the growth of Japanese forest tree species is required.
Ozone enters the leaf through the stomata and then injures cellular components such as plasma membrane (Nouchi, 2002). Since the real impacts of O3 mainly depend on the amount of O3 reaching the sites of damage within the leaf, cumulative flux or uptake of O3 through the stomata and associated response functions are suitable for mapping and quantifying impacts of O3 at the local and regional scale (Mills et al., 2010). Therefore, atmospheric concentration-based critical level of O3 expressed as AOT40 can be used only for estimating the risk of damage. The approach based on the O3 flux into leaves or needles requires the development of mathematical models to estimate stomatal O3 uptake primarily from the knowledge of stomatal responses to environmental factors (e.g. Emberson et al., 2000a, b). At the present time, however, there is limited information on stomatal flux of O3 into the leaves or needles of Japanese forest tree species (Hoshika et al., 2009). Therefore, it is necessary to promote the research toward the modeling of stomatal flux of O3 for the final purpose of mapping and quantifying the impacts of O3 on Japanese forest tree species.
6. CONCLUSION AND PERSPECTIVES
Based on the results obtained from the experimental studies, the current levels of O3 in Japan are high enough to adversely affect growth of Japanese forest tree species with relatively high O3 sensitivity such as Japanese beech. To protect Japanese forest, therefore, we need to establish the critical level of O3, primarily using AOT40 index, for Japanese forest tree species with consideration of other abiotic environmental factors affecting the sensitivity to O3 such as N deposition from the atmosphere. In addition to the estimating the risk of damage of O3 using AOT40 index, it is necessary to quantify the impacts of O3 on Japanese forest tree species. For this purpose, it is necessary to promote the research toward the modeling of stomatal flux of O3 into the leaves or needles of Japanese forest tree species. Furthermore, Kohno et al. (2005) pointed out whether results obtained from experimental studies on the effects of O3 on the growth of Japanese forest tree species using the seedlings could be applicable to the evaluation of O3-induced adverse effects on the growth of mature trees grown under natural conditions or not. To understand and evaluate the actual impacts of O3 on the growth and physiological functions of Japanese forest tree species grown in the field, therefore, further research is required for the scaling effects of O3 from seedlings to mature forest trees.
Acknowledgments
This study was financially supported by the Ministry of the Environment, Japan through the programs of Global Environmental Research Fund (C-07, 2003-2005) and Environment Research and Technology Development Fund (B-1105).
REFERENCES
-
Aber, J.D., Nadelhoffer, K.J., Steudler, P., Melillo, J.M., (1989), Nitrogen saturation in Northern forest ecosystems, Bioscience, 39, p378-386.
[https://doi.org/10.2307/1311067]

- ADORC(Acid Deposition and Oxidant Research Center), (2006), Tropospheric ozone: A growing threat, ADORC (Acid Deposition and Oxidant Research Center), Niigata, Japan, p1-16.
- Aihara, K., Aso, T., Takeda, M., Koshiji, T., (2004), Actual condition of forest decline and approach (II) The phenomena of forest decline at Tanzawa Mountain in Kanagawa prefecture, Journal of Japan Society for Atmospheric Environment, 39, pA29-A39, (in Japanese).
-
Akimoto, H., (2003), Global air quality and pollution, Science, 302, p1716-1719.
[https://doi.org/10.1126/science.1092666]

-
Andersen, C.P., Wilson, R., Plocher, M., Hogsett, W.E., (1997), Carry-over effects of ozone on root growth and carbohydrate concentrations of ponderosa pine seedlings, Tree Physiology, 17, p805-811.
[https://doi.org/10.1093/treephys/17.12.805]

- Ashmore, M., (1993), Critical levels for ozone, In Critical levels of air pollutants for Europe, Ashmore, M., and Wilson, R.B., EdsEgham, U. K, p20-47.
-
Ashmore, M., Emberson, L., Karlsson, P.E., Pleijel, H., (2004), New directions: A new generation of ozone critical levels for the protection of vegetation in Europe, Atmospheric Environment, 38, p2213-2214.
[https://doi.org/10.1016/j.atmosenv.2004.02.029]

-
Bytnerowicz, A., Godzik, B., Grodzinska, K., Fraczek, W., Musselman, R., Manning, W., Badea, O., Popescu, F., Fleischer, P., (2004), Ambient ozone in forests of the Central and Eastern European Mountains, Environmental Pollution, 130, p5-16.
[https://doi.org/10.1016/j.envpol.2003.10.032]

-
Chappelka, A.H., Samuelson, L.J., (1998), Ambient ozone effects on forest trees of the Eastern United States: A review, New Phytologist, 139, p91-108.
[https://doi.org/10.1046/j.1469-8137.1998.00166.x]

- Dentener, F., Stevenson, D., Ellingsen, K., van Noije, T., Schultz, M., Amann, M., Atherton, C., Bell, N., Bergmann, D., Bey, I., Bouwman, L., Butler, T., Cofala, J., Collins, B., Drevet, J., Doherty, R., Eickhout, B., Eskes, H., Fiore, A., Gauss, M., Hauglustaine, D., Horowitz, L., Isaksen, I.S.A., Josse, B., Lawrence, M., Krol, M., Lamarque, J.F., Montanaro, V., Müller, J.F., Peuch, V.H., Pitari, G., Pyle, J., Rast, S., Rodriguez, J., Sanderson, M., Savage, N.H., Shindell, D., Strahan, S., Szopa, S., Sudo, K., van Dingenen, R., Wild, O., Zeng, G., (2006), The global atmospheric environment for the next generation, Environmental Science and Technology, 40, p3586-3594.
- Derwent, R., Collins, W., Johnson, C., Stevenson, D., (2002), Global ozone concentrations and regional air quality, Environmental Science and Technology, 36, p379-382.
- Emberson, L.D., Wieser, G., Ashmore, M.R., (2000a), Modelling of stomatal conductance and ozone flux of Norway spruce: comparison with field data, Environmental Pollution, 109, p393-402.
- Emberson, L.D., Ashmore, M.R., Cambridge, H.M., Simpson, D., Tuovinen, J.-P., (2000b), Modelling stomatal ozone flux across Europe, Environmental Pollution, 109, p403-413.
- Emberson, L.D., Ashmore, M.R., Murray, F., Kuylenstierna, J.C.I., Percy, K.E., Izuta, T., Zheng, Y., Shimizu, H., Sheu, B.H., Liu, C.P., Agrawal, M., Wahid, A., Abdel-Latif, N.M., van Tienhoven, A.M., de Bauer, L.I., Domingos, M., (2001), Impacts of air pollutants on vegetation in developing countries, Water, Air, & Soil Pollution, 130, p107-118.
- EPA(Environmental Protection Agency), (2006a), Air quality criteria for ozone and related photochemical oxidants Vol. I, U.S. Environmental Protection Agency, Research Triangle Park, NC, USA, pE-1-34.
- EPA(Environmental Protection Agency), (2006b), Air quality criteria for ozone and related photochemical oxidants Vol. III, U.S. Environmental Protection Agency, Research Triangle Park, NC, USA, pAX9-87-155.
- Feller, U., (2004), Proteolysis, In Plant Cell Death Processes, Noodén, L.D., Eds.Elsevier Science, California, USA, p107-124.
-
Fujinuma, Y., Furukawa, A., Totsuka, T., Tazaki, T., (1987), Uptake of O3 by various street trees, Environment Control in Biology, 25, p31-39.
[https://doi.org/10.2525/ecb1963.25.31]

- Furukawa, A., Katase, M., Ushijima, T., Totsuka, T., (1983), Inhibition of photosynthesis of poplar species by ozone, Journal of the Japanese Forestry Society, 65, p321-326.
-
Furukawa, A., (1991), Inhibition of photosynthesis of Populus euramericana and Helianthus annuus by SO2, NO2 and O3, Ecological Research, 6, p79-86.
[https://doi.org/10.1007/bf02353871]

- Galloway, J.N., Aber, J.D., Erisman, J.W., Seitzinger, S.P., Howarth, R.W., Cowling, E.B., Cosby, B.N., (2003), The nitrogen cascade, Bioscience, 53, p341-356.
- Galloway, J.N., Dentener, F.J., Capone, D.G., Boyer, E.W., Howarth, R.W., Seitzinger, S.P., Asner, G.P., Cleveland, C.C., Green, P.A., Holland, E.A., Karl, D.M., Michaels, A.F., Porter, J.H., Townsend, A.R., Vörösmarty, C.J., (2004), Nitrogen cycles: past, present, and future, Biogeochemistry, 70, p153-226.
- Hoshika, Y., Hajima, T., Shimizu, Y., Takigawa, M., Omasa, K., (2009), Effect of growing season on ozone stomatal flux for deciduous forests in East Asia, Eco-Engineering, 21, p3-8.
- IPCC (Intergovernmental Panel on Climate Change), (2001), Climate change 2001: the scientific basis, University Press, Cambridge, p196.
-
Izuta, T., (1998), Ecophysiological responses of Japanese forest tree species to ozone, simulated acid rain and soil acidification, Journal of Plant Research, 111, p471-480.
[https://doi.org/10.1007/bf02507781]

- Izuta, T., (2002), Studies on the effects of ozone and acid deposition on Japanese crop plants and forest tree species, Journal of Japan Society for Atmospheric Environment, 37, p81-95, (in Japanese with English summary).
- Izuta, T., Umemoto, M., Horie, K., Aoki, M., Totsuka, T., (1996), Effects of ambient levels of ozone on growth, gas exchange rates and chlorophyll contents of Fagus crenata seedlings, Journal of Japan Society for Atmospheric Environment, 31, p95-105.
- Izuta, T., Matsumura, H., Kohno, Y., Shimizu, H., (2001), Experimental studies on the effects of acid deposition on forest tree species, Journal of Japan Society for Atmospheric Environment, 36, p137-155, (in Japanese with English summary).
-
Karlsson, P.E., Uddling, J., Braun, S., Broadmeadow, M., Elvira, S., Gimeno, B.S., Thiec, D.L., Oksanen, E., Vandermeiren, K., Wilkinson, M., Emberson, L., (2004), New critical levels for ozone effects on young trees based on AOT40 and simulated cumulative leaf uptake of ozone, Atmospheric Environment, 38, p2283-2294.
[https://doi.org/10.1016/j.atmosenv.2004.01.027]

-
Khiem, M., Ooka, R., Hayami, H., Yoshikado, H., Huang, H., Kawamoto, Y., (2010), Process analysis of ozone formation under different weather conditions over the Kanto region of Japan using the MM5/CMAQ modelling system, Atmospheric Environment, 44, p4463-4473.
[https://doi.org/10.1016/j.atmosenv.2010.07.038]

- Klimont, Z., Cofala, J., Schöpp, W., Amann, M., Streets, D.G., Ichikawa, Y., Fujita, S., (2001), Projections of SO2, NOx, NH3 and VOC emissions in East Asia up to 2030, Water, Air, & Soil Pollution, 130, p193-198.
- Kohno, Y., Matsumura, H., Ishii, T., Izuta, T., (2005), Establishing critical levels of air pollutants for protecting East Asian vegetation-A challenge, In Plant responses to air pollution and global change, Omasa, K., Nouchi, I., and De Kok, L.J., EdsSpringer-Verlag, Tokyo, p243-250.
- Kohno, Y., Suto, H., Ishii, T., Aihara, K., Uchiyama, Y., (2007), Concentration and AOT40 of ozone in Tanzawa Mountains and its potential effect on Japanse beech forests, In Results of the Scientific Research on the Tanzawa Mountains (The Research Group of the Tanzawa Mountains Eds), Hiraoka Environmental Science Laboratory, Sagamihara, Kanagawa, Japan, p383-395, (in Japanese with English summary).
-
Kume, A., Numata, S., Watanabe, K., Honoki, H., Nakajima, H., Ishida, M., (2009), Influence of air pollution on the mountain forests along the Tateyama-Kurobe Alpine route, Ecological Research, 24, p821-830.
[https://doi.org/10.1007/s11284-008-0557-2]

- Kuno, H., (1979), Effects of photochemical oxidant on the growth of poplar cuttings I, Journal of Japan Society for Air Pollution, 14, p265-274, (in Japanese with English summary).
- Kuno, H., (1980), Effects of photochemical oxidant on the growth of poplar cuttings II, Journal of Japan Society for Air Pollution, 15, p155-162, (in Japanese with English summary).
- Maruta, E., Shima, K., Horie, K., Aoki, M., Dokiya, Y., Izuta, T., Totsuka, T., Yokoi, Y., Sakata, T., (1999), Forest decline of Fagus crenata at Mt. Hinokiboramaru (Tannzawa, Kanagawa Prefecture, Japan) and acid deposition, Environmental Sciences, 12, p241-250, (in Japanese).
- Matsumoto, Y., Maruyama, Y., Morikawa, Y., Inoue, T., (1992), Some negative results of simulative acid mist and ozone treatments to Cryptomeria japonica D. Don seedlings in explanation of mature C. japonica decline in the Kanto plains in Japan, Japanese Journal for Forest Environment, 34, p85-97, (in Japanese with English summary).
- Matsumura, H., (2001), Impacts of ambient ozone and/or acid mist on the growth of 14 tree species: an open-top chamber study conducted in Japan, Water, Air, & Soil Pollution, 130, p959-964.
- Matsumura, H., Aoki, H., Kohno, Y., Izuta, T., Totsuka, T., (1996), Effects of ozone on dry weight growth and gas exchange rate of Japanese cedar, Japanese cypress and Japanese zelkova seedlings, Journal of Japan Society for Atmospheric Environment, 31, p247-261, (in Japanese with English summary).
- Matsumura, H., Kobayashi, T., Kohno, Y., (1998), Effects of ozone and/or simulated acid rain on dry weight growth and gas exchange rates of Japanese cedar, Nikko fir, Japanese white birch and Japanese zelkova seedlings, Journal of Japan Society for Atmospheric Environment, 33, p16-35, (in Japanese with English summary).
- Matsumura, H., Kohno, Y., (1999), Impact of O3 and/or SO2 on the growth of young trees of 17 tree species: an open-top chamber study conducted in Japan, In Critical Levels for Ozone-Level II, Environmental Documentation No. 115, Fuhrer, J., and Achermann, B., EdsSwiss Agency for Environment, Forest and Landscape (SAFEL), Bern, Switzerland, p187-192.
- Matsumura, H., Mikami, C., Sakai, Y., Murayama, K., Izuta, T., Yonekura, T., Miwa, M., Kohno, Y., (2005), Impacts of elevated O3 and/or CO2 on growth of Betula platyphylla, Betula ermanii, Fagus crenata, Pinus densiflora and Cryptomeria japonica seedlings, Journal of Agricultural Meteorology, 60, p1121-1124.
- Matsushima, J., Kawai, T., Oodaira, T., Sawada, T., Nouchi, I., (1977), Comparisons of fine structures of zelkova leaves with no visual injury fumigated with ozone, nitrogen dioxide, sulfur dioxide and ethylene, Journal of Japan Society for Air Pollution, 11, p360-369, (in Japanese with English summary).
- Mills, G., Pleijel, H., Büker, P., Braun, S., Emberson, L., Harmens, H., Hayes, F., Simpson, D., Grünhage, L., Karlsson, P.-E., Danielsson, H., Bermejo, V., Fernandez, I.G., (2010), Mapping critical levels for vegetation-Revision undertaken in summer, 2010, to include new flux-based critical levels and response functions for ozone, In Mapping Manual 2004, Spranger, T., EdUNECE Convention on Long-range Transboundary Air Pollution, pIII-4-57.
- Miwa, M., Izuta, T., Totsuka, T., (1993), Effects of simulated acid rain and/or ozone on the growth of Japanese cedar seedlings, Journal of Japan Society for Air Pollution, 28, p279-287, (in Japanese with English summary).
- Nakaji, T., Izuta, T., (2001), Effects of ozone and/or excess soil nitrogen on growth, needle gas exchange rates and Rubisco contents of Pinus densiflora seedlings, Water, Air, & Soil Pollution, 130, p971-976.
-
Nakaji, T., Kobayashi, T., Kuroha, M., Omori, K., Matsumoto, Y., Yonekura, T., Watanabe, K., Utriainen, J., Izuta, T., (2004), Growth and nitrogen availability of red pine seedlings under high nitrogen load and elevated ozone, Water, Air, & Soil Pollution: Focus, 4, p277-287.
[https://doi.org/10.1023/b:wafo.0000028360.61672.8d]

- Network Center for EANET, (2011), Acid Deposition Monitoring Network in East Asia (EANET) Data report 2009, Network Center for EANET, Niigata, Japan, p15-206.
- Nihlgård, B., (1985), The ammonium hypothesis-an additional explanation to the forest dieback in Europe, AMBIO, 14, p2-8.
- Nouchi, I., (2002), Responses of whole plants to air pollutants, In Air pollution and plant biotechnology-Prospects for phytomonitoring and phytoremediation, Omasa, K., Saji, H., Youssefian, S., and Kondo, N., EdsSpringer-Verlag Tokyo, Tokyo, Japan, p3-39.
- Nouchi, I., Odaira, T., (1973a), Influence of ozone on plant pigments, Journal of Japan Society for Air Pollution, 8, p120-125, (in Japanese with English summary).
- Nouchi, I., Odaira, T., Sawada, T., Oguchi, K., Komeji, T., (1973b), Plant ozone injury symptoms, Journal of Japan Society for Air Pollution, 8, p113-119, (in Japanese with English summary).
-
Ohara, T., Akimoto, H., Kurokawa, J., Horii, N., Yamaji, K., Yan, X., Hayasaka, T., (2007), An Asian emission inventory of anthropogenic emission sources for the period 1980-2020, Atmospheric Chemistry and Physics Discussions, 7, p6843-6902.
[https://doi.org/10.5194/acp-7-4419-2007]

- Oksanen, E., Saleem, A., (1999), Ozone exposure results in various carry-over effects and prolonged reduction in biomass in birch (Betula pendula Roth), Plant, Cell and Environment, 22, p1401-1411.
-
Paoletti, E., Schaub, M., Matyssek, R., Wieser, G., Augustaitis, A., Bastrup-Birk, A.M., Bytnerowicz, A., Günthardt-Goerg, M.S., Müller-Starck, G., Serengil, Y., (2010), Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services, Environmental Pollution, 158, p1986-1989.
[https://doi.org/10.1016/j.envpol.2009.11.023]

-
Pearson, M., Mansfield, T.A., (1994), Effects of exposure to ozone and water stress on the following season’s growth of beech (Fagus sylvatica L.), New Phytologist, 126, p511-515.
[https://doi.org/10.1111/j.1469-8137.1994.tb04249.x]

-
Richter, A., Burrows, J.P., Nüβ, H., Granier, C., Niemeier, U., (2005), Increase in tropospheric nitrogen dioxide over China observed from space, Nature, 437, p129-132.
[https://doi.org/10.1038/nature04092]

- Sandermann, H., Wellburn, A.R., Heath, R.L., (1997), Forest decline and Ozone: Synopsis, In Forest decline and Ozone, Sandermann, H., Wellburn, A.R., and Heath, R.L., EdsSpringer-Verlag Berlin Heidelberg, Germany, p369-377.
-
Schulze, E.-D., (1989), Air pollution and forest decline in a spruce (Picea abies) forest, Science, 244, p776-783.
[https://doi.org/10.1126/science.244.4906.776]

- Skärby, L., Ro-Poulsen, H., Wellburn, F.A.M., Sheppard, L.J., (1998), Impacts of ozone on forests: a European perspective, New Phytologist, 139, p109-122.
-
Spreitzer, R.J., Salvucci, M.E., (2002), Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme, Annual Review of Plant Biology, 53, p449-475.
[https://doi.org/10.1146/annurev.arplant.53.100301.135233]

-
Suto, H., Hattori, Y., Tanaka, N., Kohno, Y., (2008), Effects of strong wind and ozone on localized tree decline in the Tanzawa Mountains of Japan, Asian Journal of Atmospheric Environment, 2, p81-89.
[https://doi.org/10.5572/ajae.2008.2.2.081]

- Takeda, M., Aihara, K., (2007), Effects of ambient ozone concentrations on beech (Fagus crenanta) seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan, Journal of Japan Society for Atmospheric Environment, 42, p107-117, (in Japanese with English summary).
-
Vitousek, P.M., Howarth, R.W., (1991), Nitrogen limitation on land and in the sea: How can it occur?, Biogeochemistry, 13, p87-115.
[https://doi.org/10.1007/bf00002772]

- Watanabe, M., Yonekura, T., Honda, Y., Yoshidome, M., Nakaji, T., Izuta, T., (2005), Effects of ozone and soil water stress, singly and in combination, on leaf antioxidative systems of Fagus crenata seedlings, Journal of Agricultural Meteorology, 60, p1105-1108.
- Watanabe, M., Yamaguchi, M., Iwasaki, M., Matsuo, N., Naba, J., Tabe, C., Matsumura, H., Kohno, Y., Izuta, T., (2006), Effects of ozone and/or nitrogen load on the growth of Larix kaempferi, Pinus densiflora and Cryptomeria japonica seedlings, Journal of Japan Society for Atmospheric Environment, 41, p320-334.
-
Watanabe, M., Yamaguchi, M., Tabe, C., Iwasaki, M., Yamashita, R., Funada, R., Fukami, M., Matsumura, H., Kohno, Y., Izuta, T., (2007), Influences of nitrogen load on the growth and photosynthetic responses of Quercus serrata seedlings to O3, Trees, 21, p421-432.
[https://doi.org/10.1007/s00468-007-0134-2]

-
Watanabe, M., Yamaguchi, M., Matsumura, H., Kohno, Y., Izuta, T., (2008), Effects of ozone on the growth and photosynthesis of Castanopsis sieboldii seedlings grown under different nitrogen loads, Journal of Agricultural Meteorology, 64, p143-155.
[https://doi.org/10.2480/agrmet.64.3.6]

-
Watanabe, M., Umemoto-Yamaguchi, M., Koike, T., Izuta, T., (2010), Growth and photosynthetic response of Fagus crenata seedlings to ozone and/or elevated carbon dioxide, Landscape and Ecological Engineering, 6, p181-190.
[https://doi.org/10.1007/s11355-009-0095-2]

- Yamaguchi, M., Watanabe, M., Matsuo, N., Naba, J., Funada, R., Fukami, M., Matsumura, H., Kohno, Y., Izuta, T., (2007a), Effects of nitrogen supply on the sensitivity to O3 of growth and photosynthesis of Japanese beech (Fagus crenata) seedlings, Water, Air, & Soil Pollution: Focus, 7, p131-136.
- Yamaguchi, M., Watanabe, M., Iwasaki, M., Tabe, C., Matsumura, H., Kohno, Y., Izuta, T., (2007b), Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads, Trees, 21, p707-718.
-
Yamaguchi, M., Watanabe, M., Matsumura, H., Kohno, Y., Izuta, T., (2010), Effects of ozone on nitrogen metabolism in the leaves of Fagus crenata seedlings under different soil nitrogen loads, Trees, 24, p175-184.
[https://doi.org/10.1007/s00468-009-0391-3]

-
Yamaji, K., Ohara, T., Uno, I., Tanimoto, H., Kurokawa, J., Akimoto, H., (2006), Analysis of the seasonal variation of ozone in the boundary layer in East Asia using the Community Multi-scale Air Quality model: What controls surface ozone levels over Japan?, Atmospheric Environment, 40, p1856-1868.
[https://doi.org/10.1016/j.atmosenv.2005.10.067]

-
Yamaji, K., Ohara, T., Uno, I., Kurokawa, J., Pochanart, P., Akimoto, H., (2008), Future prediction of surface ozone over east Asia using Models-3 Community Multiscale Air Quality Modeling System and Regional Emission Inventory in Asia, Journal of Geophysical Research, 113, D08306.
[https://doi.org/10.1029/2007jd008663]

- Yonekura, T., Dokiya, Y., Fukami, M., Izuta, T., (2001a), Effects of ozone and/or soil water stress on growth and photosynthesis of Fagus crenata seedlings, Water, Air, & Soil Pollution, 130, p965-970.
- Yonekura, T., Honda, Y., Oksanen, E., Yoshidome, M., Watanabe, M., Funada, R., Koike, T., Izuta, T., (2001b), The influences of ozone and soil water stress, singly and in combination, on leaf gas exchange rates, leaf ultrastructural characteristics and annual ring width of Fagus crenata seedlings, Journal of Japan Society for Atmospheric Environment, 36, p333-351.
-
Yonekura, T., Yoshidome, M., Watanabe, M., Honda, Y., Ogiwara, I., Izuta, T., (2004), Carry-over effects of ozone and water stress on leaf phenological characteristics and bud frost hardiness of Fagus crenata seedlings, Trees, 18, p581-588.
[https://doi.org/10.1007/s00468-004-0345-8]

