A Study on Three Factors Influencing Uptake Rates of Nitric Acid onto Dust Particles
Abstract
Recent studies have indicated that the observed nitric acid (HNO3) uptake rates (RHNO3) onto dust particles are much slower than RHNO3 used in the previous modeling studies. Three factors that possibly affect RHNO3 onto dust particles are discussed in this study: (1) the magnitude of reaction probability of HNO3 (γHNO3), (2) aerosol surface areas, and (3) gas-phase HNO3 mixing ratio. Through the discussion presented here, it is shown that the use of accurate γHNO3 is of primary importance. We suggest that the use of γHNO3 values between ~10-3 and ~10-5 produces more realistic results than the use of γHNO3 values between ~10-1 and ~10-2 does, more accurately modeling the nitrate formation characteristics on/in dust particles. We also discuss two different types of aerosol surface area, active and geometric, since the use of different aerosol surface areas often leads to an erroneous result in RHNO3. In addition, the levels of the gas-phase HNO3 are investigated with the example cases of TRACE-P DC-8 flights in East Asia. The HNO3 levels were found to be relatively high, indicating that they can not limit nitrate formation in dust particles.
Keywords:
Reaction probability, Dust particles, Nitric acid, Uptake rates, Aerosol surface area1. INTRODUCTION
Recently, several studies have reported that although East Asian dust particles have sufficient chemical aging (or contacting) times (say, 1-4 days) with atmospheric air pollutants, they were found to contain only small amounts of nitrate (and/or sulfate) (Song et al., 2007; Song et al., 2005; Maxwell-Meier et al., 2004). This fact possibly indicates that the gas-to-particle nitric acid (HNO3) uptake rates (RHNO3) onto dust particles are slower than previously estimated. This could also be an important correction to the results from previous studies (Meskhidze et al., 2003; Song and Carmichael, 2001; Zhang and Carmichael, 1999; Dentener et al., 1996). For example, Meskhidze et al. (2003) inferred from the data of TRACE-P DC-8 Flight #13 that total nitrate (=HNO3(g)+NO3-(p)) distribution between gas phase and East Asian dust particles reaches a near equilibrium (refer to Fig. 4 in Meskhidze et al. (2003)). Their work supported the opposite idea that dust particles could contain a quite large amount of nitrate that could completely neutralize dust-originated cationic components such as Ca2+ and Mg2+. This study, therefore, intends to discuss possible causes of this discrepancy in order to offer a convincing explanation.
Closely connected with this issue, controversy has continued regarding the magnitude of reaction probability of HNO3 (γHNO3) onto dust particles. Several research groups measured γHNO3 onto various types of dust particle, from proxy species of dust particles (e.g., α-Al2O3, SiO2, CaCO3 etc) to authentic Saharan and Gobi dust. However, a large difference in the magnitude of γHNO3 onto dust particles has been reported (Johnson et al., 2005; Umann et al., 2005; Harnisch and Crowley, 2001a, b; Underwood et al., 2001a, b; Fenter et al., 1995), as presented in Table 1. Here, the first group of γHNO3 has an order of magnitude from ~10-1 to ~10-2 (Umann et al., 2005; Harnisch and Crowley, 2001a, b; Fenter et al., 1995), compared to a range from ~10-3 to ~10-5 for the second group (Johnson et al., 2005; Underwood et al., 2001a, b).
The ultimate purpose of estimating (or measuring) γHNO3 is to apply the result to the chemistry-transport modeling studies. However, because of the large differences in the magnitude of γHNO3 onto dust particles, atmospheric modelers have been confused about which value should be adopted in their modeling studies (Song et al., 2007; Bauer et al., 2004; Zhang and Carmichael, 1999; Dentener et al., 1996; and also the third group of γHNO3 as presented in Table 1). In addition, it appears that the parameterizations (or estimation method for γHNO3) for describing the heterogeneous interaction between HNO3 and dust particles are questionable. Therefore, an impartial discussion is urgently required about both the magnitudes of γHNO3 onto dust particles and the parameterizations for the heterogeneous interactions between HNO3 and dust particles. In this study, we discuss all the relevant and yet contentious issues regarding the heterogeneous processes between gasphase HNO3 and dust particles. In addition, through this study we wish to provide a modeler’s perspective regarding the chemical evolution of dust particles and the magnitude of γHNO3 onto dust particles.
2. DISCUSSION
2. 1 Research and Theoretical Background
The HNO3 uptake into dust particles causes the replacement of carbonate (CO32-) originally associated with crustal Ca2+ (and/or Mg2+) in dust particles via the following reaction:
| (R-1) |
Similar carbonate replacement reactions occur with the uptake of other acidic substances such as H2SO4 and SO2. Based on this mechanism, Table 2 presents the “dust chemical aging index”, defined as the ratio of estimated CO32- equivalence ([CO32-] in μeq/m3) to crustal cation equivalence ([Ca2+]+[Mg2+]) at several different locations in East Asia (Song et al., 2007; 2005). As presented in Table 2, the ratios range from 0.39 to 0.87, indicating that the remaining carbonate fractions in the dust particles range from 39-87%, respectively, even after the chemical aging times of 24-84 hrs. Several single particle chemical analysis studies with East Asian dust particles reached the same conclusions (Ro et al., 2005; Zhang et al., 2003; Zhang and Iwasaka, 1999). The replaced CO32- fractions of 13-61% are believed to be released from dust particles by nitrate (and/or sulfate) formation. The small percentages of the replaced carbonate, even with the long chemical aging times, are obvious evidences of slow RHNO3 onto dust particles.
Assuming pseudo first-order kinetics (eqn. 1), RHNO3 can be calculated by eqns. (1) and (2). In the equations, the magnitude of RHNO3 can be affected by three factors: i) γHNO3 ii) aerosol surface density (Sa); and iii) gasphase HNO3 concentration (CHNO3):
| (1) |
| (2) |
where k denotes the mass transfer coefficient (1/s), vHNO3 the molecular mean velocity of HNO3 (cm/s), and Sa the aerosol surface density (μm2/cm3). In this study, we discuss these three possibly influential factors to determine which factor (or factors) is really responsible for the observed discrepancy in RHNO3 onto dust particles.
2. 2 Reaction Probability of HNO3 (γHNO3)
First of all, the slow RHNO3 could result from low γHNO3 onto dust particles. As mentioned in the Introduction, large differences have been reported in the magnitude of γHNO3 onto dust particles (Song et al., 2007; Johnson et al., 2005; Umann et al., 2005; Harnisch and Crowley, 2001a, b; Underwood et al., 2001a, b; Fenter et al., 1995). Fig. 1 presents how fast HNO3 can be transferred from the gas phase into dust particles with different γHNO3 values, using eqns. (1) and (2). For example, when γHNO3=0.1, the 10 e-folding lifetime (τ10, defined as the time at which CHNO3/CHNO3,0=1/10e=0.037; τ10=(1+ln10)/k, where k represent the mass transfer coefficient in eqn. (2)) is only 1.41 hrs. When γHNO3=0.01, τ10 becomes 8.03 hrs. In other words, 96.3% of HNO3 is partitioned into the particulate phase within 8.03 hrs, when γHNO3=0.01. The partitioned HNO3 is then rapidly converted into nitrate, being associated with Ca2+ and Mg2+ ions inside the dust particles. For example, if the HNO3 levels of 1-3 ppb are converted into nitrate at STP at a yield of 96.3%, nitrate concentrations of 2.6-7.7 μg/m3 should be found in the dust particles after relatively short chemical aging times (within few hours). Once these amounts of nitrate are formed in the dust particles, the particles (particularly Ca2+) can be completely neutralized only by nitrate in most typical dusty situations. HNO3 levels of 1-3 ppb were frequently observed with high dust concentrations over the downwind areas from the polluted regions in East Asia (this will be discussed in more detail in section 2.4; also refer to Bauer et al. (2004)). However, such large amounts of nitrate have never been found in dust particles, particularly in East Asia (Song et al., 2007; Ro et al., 2005; Song et al., 2005; Zhang et al., 2003). In contrast, when γHNO3= 10-3-10-5, RHNO3 become much slower (τ10=3.1-91.8 days). With the initial CHNO3 of 1-3 ppb and γHNO3 of 10-4, the converted amount of nitrate after the interacting times of 8 hours is 0.095-0.29 μg/m3, which is equivalent to an hourly averaged RHNO3 of 0.01-0.04 μg/m3∙hr. Such slow RHNO3 are more consistent with the slow chemical evolution of dust particles observed in the field measurements. For example, in Table 2, we estimated the possibly maximum nitrate concentrations (; ). The measurement data used in Table 2 were obtained from the ACE-ASIA C130 flight campaign conducted over the Yellow sea on April 11-13, 2001, and a measurement campaign conducted in Seoul in April, 2005. We selected the data from the dust storm periods of the campaigns. In this calculation, while we cannot estimate the individual amounts of nitrate and sulfate, we can estimate the combined amounts of “nitrate+sulfate” concentration in the dust particles by equating the replaced carbonate equivalences to the “nitrate+sulfate” equivalences. In an extreme case, carbonate in dust particles could be replaced only by NO3-. These amounts (). are then divided by chemical aging times to obtain the maximum possible nitrate uptake rates. As shown in Table 2, the rates range from 0.01 to 0.26 μg/m3∙hr. These values are in general comparable to the estimated rates above (0.01-0.04 μg/m3∙hr), particularly for the cases of ACE-ASIA C-130 Flights #6, #7, and #8 which recorded results in the range 0.05-0.07 μg/m3∙hr (again, it is important to remember that the average uptake rates actually represent the production rates of NO3-+SO42-).
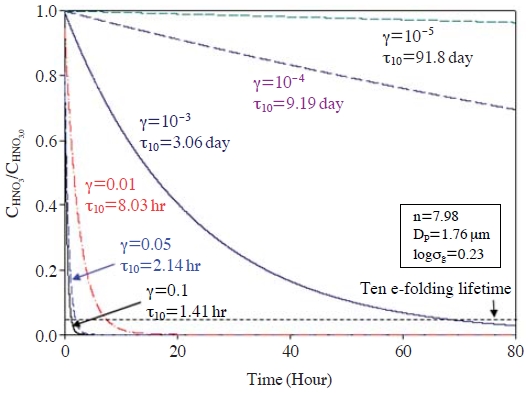
Ten e-folding lifetimes (τ10) with different reaction probability of HNO3. The parameters for log-normal aerosol distribution used in this figure are presented in the box (Zhang et al., 1994).
Although in this study we do not intend to estimate γHNO3 onto dust particles, one of the objectives of this study is to decide which values of “already-measured” γHNO3 in Table 1 can be adopted in the dust chemistry modeling studies. Based on the calculations above, the modeling studies with γHNO3 of ~10-3-~10-5 could produce more consistent results with the field measurement data.
2. 3 Surface Area
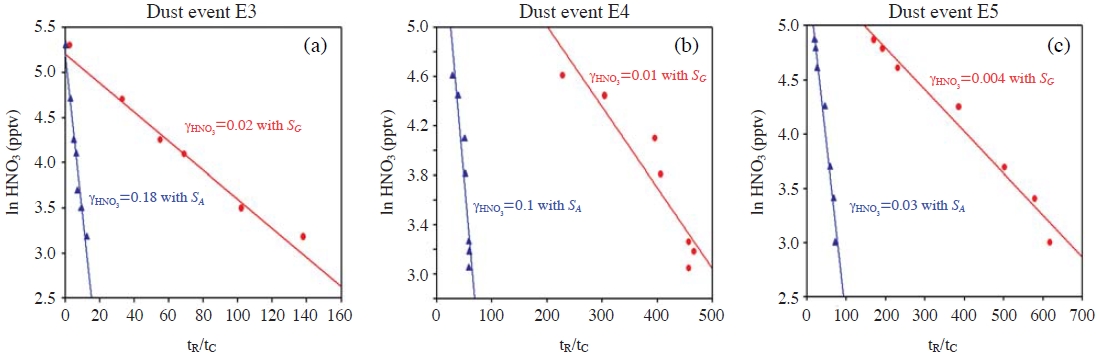
There is another possible mechanism that could slow down RHNO3 into dust particles: the application of a smaller surface area to eqn. (2). For example, in an estimating procedure of γHNO3, Umann et al. (2005) introduced an “active surface area (SA)” (for greater detail, refer to both Umann et al. (2005) and Matter Engineering, Appendix IV of Operating Instructions LQ1-DC, SKM990318-7b). A similar type of active surface area, so-called “Fuchs surface”, was also introduced by Pandis et al. (1991) and Shi et al. (2001). Whichever active surface area is used, SA has a tendency to become smaller than the “geometric surface area”) (SG), when the coarse-mode fraction is large, such as in dust and sea-salt aerosol cases. Therefore, if we use SA instead of SG for the gas-to-particle mass transfer process, RHNO3 could become slow. Table 3 presents the typical ratios of SA to SG for dust and seasalt particle distributions (Sander and Crutzen, 1996; Zhang et al., 1994; Jaenicke, 1993). The ratios range between 0.11 and 0.41. In particular the ratio is 0.11 for the two typical dust cases, indicating that RHNO3 become slower by a factor of 0.11, even if the same γHNO3 is used. Here, the relevant question is whether SA can be applied to eqn. (2). Umann et al. (2005) used SA with the concept of the “actual surface area” that is accessible for impinging gas molecules that can typically be measured by a BET (Brunauer, Emmett and Teller) type of instrument. However, both the active (μm2/cm3) and Fuchs (1/cm3) surface areas are not the “actual/accessible surface area”, but an imaginary aerosol-surface area conveniently adjusted to consider the changing mechanism in the gas-to-particle mass transfer in accordance with the aerosol size changes (Pandis et al., 1991). For the fine particles, the gas-to-particle mass transfer (uptake) rates are proportional to the second moment (i.e., surface area), whereas for the coarse particles, RHNO3 are proportional to the first moment (i.e., radius). Therefore, the SA-to-SG ratios are small when the coarse aerosol fraction is large, whereas the ratios are close to unity when the fine fraction is dominant. The variation in uptake mechanism with the aerosol size can be taken into account by the use of Fuchs and Sutugin kinetics (1971) or other similar kinetics (e.g., see p. 604 in Seinfeld and Pandis, 1998). In Table 3, the Fuchs-Sutugin mass transfer coefficient (kF) is compared with kA (k from eqn. 2 with SA for Sa) and kG (k from eqn. 2 with SG for Sa). As shown in Table 3, the values of kG are consistent with kF, whereas the values of kA are smaller than kF by a factor of ~0.1. Meanwhile, the use of SA in eqn. (2) also leads to an erroneous estimation of γHNO3. One example of the erroneous results is shown in Fig. 2. Umann et al. (2005) estimated γHNO3 from the field measurements in Sahara desert, using equations (1) and (2) with SA. We selected three dust episodes (E3, E4, and E5) from Umann et al.’s work (2005), and then re-estimated γHNO3 with SA and SG. As shown in Fig. 2, when SG is used instead of SA, the values of γHNO3 are decreased down to 0.004-0.02, which are smaller than Umann et al.’s values (γHNO3=0.03-0.18). These values are also closer to the second group of γHNO3 in Table 1 that we recommended to be used in the future dust chemistry modeling studies.
2. 4 HNO3 Concentration in the Gas Phase (CHNO3)
The third factor that could affect RHNO3 into dust particles is CHNO3 (from eqn. 1). If CHNO3 is low, RHNO3 becomes slow. Or, if the amounts of HNO3 are not sufficient, the amounts of nitrate formed in dust particles can be limited by the insufficient the amounts of HNO3, even with fast RHNO3. Particularly, extremely low CHNO3 could occur over the areas where NH3 concentrations are high. Over such areas, HNO3 can be depleted by the reaction of HNO3(g)+NH3(g) → NH4NO3(aerosol). For example, East Asia has large NH3 emissions (Kim et al., 2006). Fig. 3 presents CHNO3 and the Ca2+ concentrations measured by TRACE-P DC-8 Flights #9 and #13 over the Yellow Sea and East China Sea (see panels (c) and (g)). As presented in panels (d) and (h), the air masses originated from the Gobi desert and the arid areas in Inner Mongolia, and then passed through the highly polluted, high NOx and NH3 emission areas in China such as Beijing, Tianjin, Qingdao, Dalian, Nanjing, and Shanghai, as well as various Chinese agricultural areas (Kim et al., 2006). The high levels of Ca2+ indicate that the air masses intercepted by DC-8 Flights #9 and #13 contained high levels of dust particles. In the two flights, the observed levels of HNO3 were increased up to as high as 7 ppb (see panel (g)). The typical HNO3 levels reported from other TRACE-P DC-8 and P3-B flights in the boundary layer under the continental outflow situations ranged between ~1 ppb and ~3 ppb. Despite the coexistence of the high levels of dust particles and HNO3, Song et al. (2007; 2005) reported very low nitrate (and/or sulfate) concentrations inside dust particles over the areas close to the flight paths of TRACE-P DC-8 Flights #9 and #13. This is an important correction to the results from Meskhidze et al. (2003). They insisted that the coexistence of high levels of Ca2+ and HNO3 in the TRACE-P DC-8 Flight #13 was firm evidence that HNO3 had already filled up the dust particles (i.e., complete neutralization of Ca2+or 100% carbonate replacement had occurred).
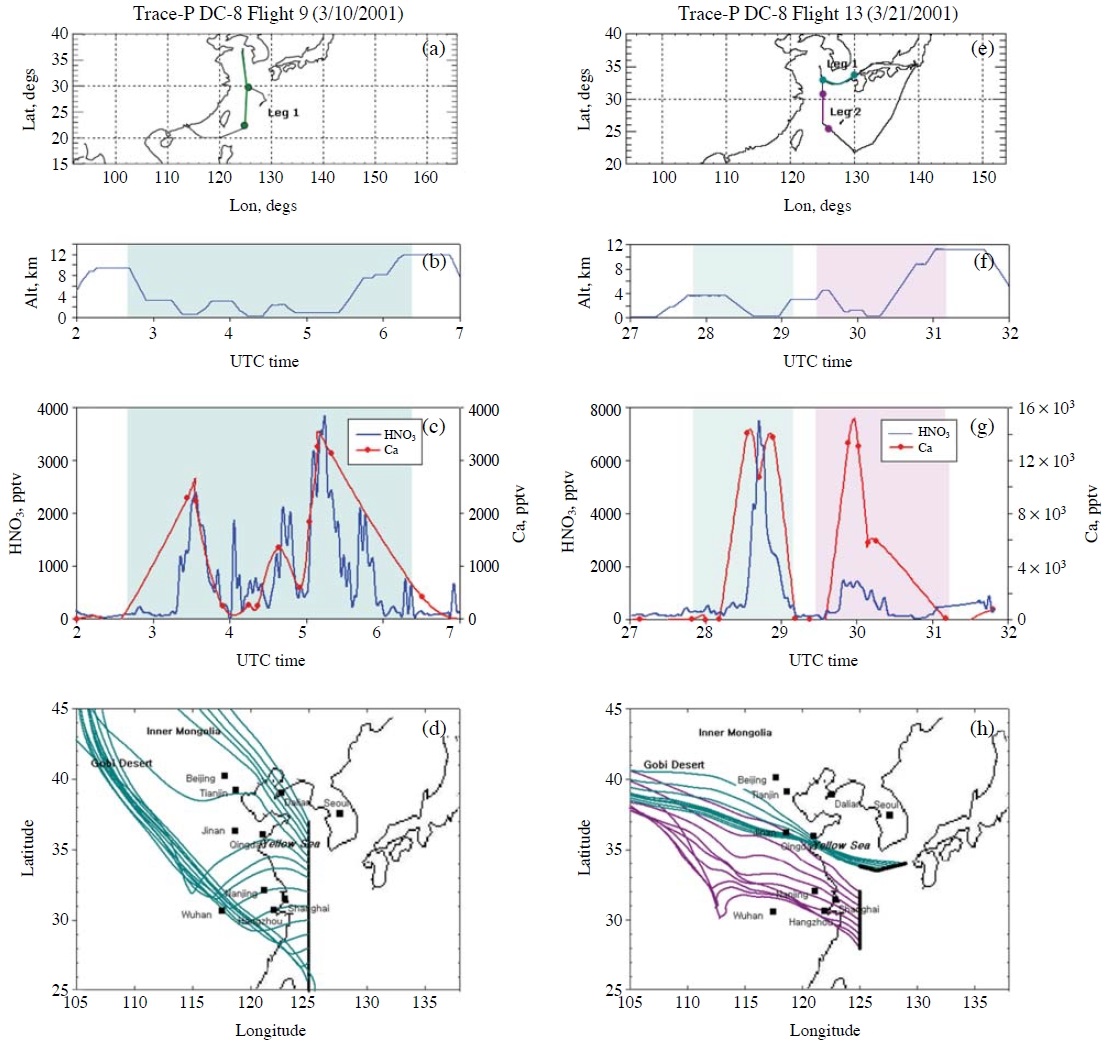
The HNO3 and Ca2+ concentrations encountered by TRACE-P DC-8 Flights #9 and #13: (a) and (e) fight paths; (b) and (f) altitudes of the flights; (c) and (g) HNO3 and Ca2+ concentrations; and (d) and (h) five-day backward trajectory analyses, respectively, using the HYSPLIT model and meteorological data available on the NOAA/ARL web site (Draxler and Hess, 1998).
Again, the presence of high HNO3 levels indicates that the low nitrate concentrations in the dust particles were not a result of the low CHNO3 levels, but were rather due to the small γHNO3 that was reduced even smaller than 10-3.
3. CONCLUSIONS
The observed RHNO3 onto dust particles were much slower than previously estimated. In addition, RHNO3 have been overestimated in several previous dust modeling studies, for which three possibilities were discussed here: (1) the magnitude of γHNO3, (2) aerosol surface area, and (3) CHNO3. Regarding the second factor, we suggested that SG should be used in eqn. (2) instead of SA, which had been used, for example, in Umann et al.’s study (2005). With respect to the third factor, we showed that the observed CHNO3 was relatively high, sometimes increasing up to as high as ~7 ppb in the marine boundary layer in East Asia, for example. CHNO3 can not limit nitrate formation in dust particles.
The over-predicted RHNO3 onto the dust particles may have been caused by the first factor-overestimated γHNO3 values (γHNO3=10-1-10-2). In the previous modeling studies of Dentener et al. (1996), Zhang and Carmichael (1999), and Bauer et al. (2004), γHNO3 values of 0.1 and 0.01 were used. In contrast to these overestimated γHNO3 values, smaller γHNO3 values within the range 10-3-10-5 have also been reported by Underwood et al. (2001a, b) and Johnson et al. (2005). In this study we concluded that the use of γHNO3 values between ~10-3 and ~10-5 could produce more realistic results than those within the range of 10-1-10-2 could, by more accurately predicting the nitrate formation characteristics in dust particles.
Acknowledgments
This work was financially supported by the Mid-Career Research Program, through a National Research Foundation of Korea (NRF) grant from the Ministry of Education, Science and Technology (MEST) (2010-0014058); and a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. R17-2008-042-01001-0).
REFERENCES
-
Bauer, S.E., Balkanski, Y., Schulz, M., Hauglusteine, D., (2004), Global modeling of heterogeneous chemistry on mineral aerosol surfaces: Influence on tropospheric ozone chemistry and comparison to observations, Journal of Geophysical Research-Atmospheres, 109, D02304.
[https://doi.org/10.1029/2003JD003868]

-
Dentener, F.J., Carmichael, G.R., Zhang, Y., Lelieveld, J., Crutzen, P.J., (1996), Role of mineral aerosol as a reactive surface in the global troposphere, Journal of Geophysical Research-Atmospheres, 101, p22869-22889.
[https://doi.org/10.1029/96jd01818]

- Draxler, R.R., Hess, G.D., (1998), An overview of the HYSPLIT_4 modeling system for trajectories, dispersion and deposition, Australian Meteorological Magazine, 47(4), p295-308.
-
Fenter, F., Caloz, F., Rossi, M.J., (1995), Experimental evidence for the efficient “Dry deposition” of nitric acid on calcite, Atmospheric Environment, 29(22), p3365-3327.
[https://doi.org/10.1016/1352-2310(95)00183-y]

- Fuchs, N.A., Sutugin, A.G., (1971), High dispersed aerosols, in Tropics in Current Aerosol Research, Hidy, G.M., and Brock, J.R., EdsPeragamon, New York, p1-200.
- Hanisch, F., Crowley, J.N., (2001a), Heterogeneous reactivity of gaseous nitric on authentic mineral dust samples, and on individual mineral and clay mineral components, Physical Chemistry Chemical Physics, 3, p2474-2482.
- Hanisch, F., Crowley, J.N., (2001b), Heterogeneous reactivity of gaseous nitric acid on Al2O3, CaCO3, and atmospheric dust samples: A Knudsen cell study, Journal of Physical Chemistry A, 105, p3096-3106.
- Jaenicke, R., (1993), Troposphere aerosols, in Aerosol-Cloud-Climate Interactions, Hobbs, P.V., Ed.Academic Press, San Diego, p1-31.
-
Johnson, E.R., Sciegienka, J., Carlos-Cuellar, S., Grassian, V.H., (2005), Heterogeneous uptake of gaseous nitric acid on dolomite (CaMg(CO3)2) and calcite (CaCO3) particles: A Knudsen cell study using multiple, single, and fractional particle layers, Journal of Physical Chemistry A, 109, p6901-6911.
[https://doi.org/10.1021/jp0516285]

- Kim, K.J., Song, C.H., Ghim, Y.S., Won, J.G., Yoon, S.C., Carmichael, G.R., Woo, J.-H., (2006), An investigation on NH3 emissions and particulate NH4+ and NO3- formation in East Asia, Atmospheric Environment, 40, p2139-2150.
-
Maxwell-Meier, K., Weber, R.J., Song, C.H., Orsini, D., Ma, Y., Carmichael, G.R., Streets, D.G., Blomquist, B., (2004), Inorganic composition of fine particles in mixed mineral dust-pollution plumes observed from airborne measurements during ACE-Asia, Journal of Geophysical Research-Atmospheres, 109, D19S07.
[https://doi.org/10.1029/2003JD004464]

-
Meskhidze, N., Chameides, W.L., Nenes, A., Chen, G., (2003), Iron mobilization in mineral dust: Can anthropogenic SO2 emissions affect ocean productivity, Geophysical Research Letters, 30(21), p2085.
[https://doi.org/10.1029/2003GL018035]

-
Pandis, S.N., Baltensperger, U., Wolfenbarger, J.K., Seinfeld, J.H., (1991), Inversion of aerosol data from the epiphanometer, Journal of Aerosol Science, 22(4), p417-428.
[https://doi.org/10.1016/0021-8502(91)90002-y]

-
Ro, C.-U., Hwang, H., Kim, H., Chun, Y., Van Grieken, R., (2005), Single particle characterization of four “Asian Dust” samples collected in Korea, using low-Z particle electron probe X-ray microanalysis, Environmental Science & Technology, 39, p1409-1419.
[https://doi.org/10.1021/es049772b]

-
Sander, R., Crutzen, P.J., (1996), Model study indicating halogen activation and ozone destruction in polluted air masses transported to the sea, Journal of Geophysical Research-Atmospheres, 101, D4.
[https://doi.org/10.1029/95JD03793]

- Seinfeld, J.H., Pandis, S.N., (1998), Atmospheric chemistry and physics, A Wiley-Interscience Publication, New York, p604.
-
Shi, J.P., Harrison, R.M., Evans, D., (2001), Comparison of ambient particle surface area measurement by epiphaniometer and SMPS/APS, Atmospheric Environment, 35, p6193-6200.
[https://doi.org/10.1016/s1352-2310(01)00382-x]

- Song, C.H., Carmichael, G.R., (2001), Gas-particle partitioning of nitric acid modulated by alkaline aerosol, Journal of Atmospheric Chemistry, 40, p1-22.
-
Song, C.H., Maxwell-Meier, K., Weber, R.J., Kapustin, V., Clarke, A., (2005), Dust composition and mixing state inferred from airborne composition measurements during ACE-Asia C130 Flight#6, Atmospheric Environment, 39, p359-369.
[https://doi.org/10.1016/j.atmosenv.2004.08.046]

-
Song, C.H., Kim, C.M., Lee, Y.J., Carmichael, G.R., Lee, B.K., Lee, D.S., (2007), An evaluation of reaction probabilities of sulfate and nitrate precursors onto East Asian dust particles, Journal of Geophysical Research-Atmospheres, 112, D18206.
[https://doi.org/10.1029/2006JD008092]

- Umann, B., Arnold, F., Schaal, C., Hanke, M., Uecker, J., Aufmhoff, H., Balkanski, Y., Van Dingenen, R., (2005), Interaction of mineral dust with gas phase nitric acid and sulfur dioxide during the MINATORIC II field campaign: First estimate of the uptake coefficient γHNO3 from atmospheric data, Journal of Geophysical Research-Atmospheres, 110, p22306-22323.
- Underwood, G.M., Li, P., Al-Abadleh, H., Grassian, V.H., (2001a), A knudsen cell study of the heterogeneous reactivity of nitric acid on oxide and mineral dust particles, Journal of Physical Chemistry A, 105, p6609-6620.
- Underwood, G.M., Song, C.H., Phadnis, M., Carmichael, G.R., Grassian, V.H., (2001b), Heterogeneous reactions of NO2 and HNO3 on oxides and mineral dust: A combined laboratory and modeling study, Journal of Geophysical Research-Atmospheres, 106, p18055-18066.
- Zhang, D., Iwasaka, Y., (1999), Nitrate and sulfate in individual Asian dust-storm particles in Beijing, China in the spring of 1995 and 1996, Atmospheric Environment, 33, p3213-3223.
- Zhang, D., Zang, J., Shi, G., Iwasaka, Y., Matsuki, A., Trochkine, D., (2003), Mixture of individual Asian dust particles at a coastal site of Qingdao, China, Atmospheric Environment, 37, p3895-3901.
-
Zhang, Y., Sunwoo, Y., Kothamarthi, V., Carmichael, G.R., (1994), Photochemical oxidant processes in the presence of dust: An evaluation of the impact of dust on particulate nitrate and ozone formation, Journal of Applied Meteorology, 33, p813-824.
[https://doi.org/10.1175/1520-0450(1994)033<0813:popitp>2.0.co;2]

-
Zhang, Y., Carmichael, G.R., (1999), The role of mineral aerosol in tropospheric chemistry in East Asia-A model study, Journal of Applied Meteorology, 38(3), p353-366.
[https://doi.org/10.1175/1520-0450(1999)038<0353:tromai>2.0.co;2]