Atmosphere-forest Exchange of Ammoniacal Nitrogen in a Subalpine Deciduous Forest in Central Japan during a Summer Week
Abstract
The present study aimed to investigate the diurnal variations in air concentrations and exchange fluxes of ammoniacal nitrogen (NHx: ammonia (NH3) and particulate ammonium) in a subalpine deciduous forest in central Japan during a week in summer. The NH3 concentrations (0.50 μg N m-3; on average) showed a clear circadian variation, i.e., high and low in the daytime and nighttime, respectively. The concentration of particulate ammonium in the coarse fractions was extremely low, whereas that for the PM2.5 fraction was relatively high (0.55 μg N m-3; on average). The main inorganic ion components of PM2.5 at the study site were ammonium and sulfate. The exchange fluxes of NHx were bidirectional. Both the maximum and minimum values occurred in the daytime, i.e., 0.39 mg N m-2 hr-1 of downward flux and 0.11 mg N m-2 hr-1 of upward flux for NH3 and 0.25 mg N m-2 hr-1 of downward flux and 0.13 mg N m-2 hr-1 of upward flux for PM2.5 ammonium. The exchange fluxes of NHx at night could be considered as zero. The mean deposition velocity during the research period was almost zero for both NH3 and PM2.5 ammonium. The atmosphere-forest exchange of NHx in the forest during the study period was balanced. The remarkably large deposition of NHx was attributable to meteorological events such as showers the night before that thoroughly washed the forest canopy and subsequent clear skies in the morning, which enhanced convection. The cleaning effect of rainfall and the rapid change in convection in the early morning should be monitored to evaluate and generalize the gas and particle exchange in a forest.
Keywords:
Ammonia, Atmosphere-forest exchange, Dry deposition, Emission, Particulate ammonium1. INTRODUCTION
Increases in nitrogen deposition induced by anthropogenic nitrogen emissions have impacts on natural ecosystems, such as acidification, eutrophication, and other related environmental consequences (Hayashi and Yan, 2010; Bouwman et al., 2002; Matson et al., 2002). In this regard, ammonia (NH3) is one of the most important forms of nitrogen because it has the largest anthropogenic emissions in the world (47.2 Tg N yr-1 in the early 1990s), which outweighs those of nitrogen oxides (36.2 Tg N yr-1) (Galloway et al., 2004). After deposition, NH3 can potentially cause acidification, via proton production that occurs during nitrification, as well as eutrophication, because NH3 is biologically available nitrogen.
Ammonia is also a main precursor of secondary inorganic particles such as particulate ammonium (pNH4) (Erisman and Schaap, 2004), which is formed by the condensation of NH3 with acids in the atmosphere. Secondary particles are mostly fine particles classified as PM2.5 (particles with a diameter less than 2.5 μm). PM2.5 can cause health risks for respiratory diseases (Neuberger et al., 2004) and can be transported long distances in the atmosphere due to their relatively long residence time (Niemi et al., 2009). PM2.5 is also related to climate change due to their radiative forcing (IPCC, 2007).
Part of pNH4 can evaporate and then release NH3. The equilibrium between the condensation and evaporation of pNH4 is affected by the chemical forms of pNH4, the air concentrations of relevant species, and temperature. In general, the dominant chemical forms of pNH4 correspond to ammonium sulfate and ammonium nitrate (e.g., Bardouki et al., 2003). For ammonium sulfate, the vapor pressure of sulfate salts is sufficiently low for the condensation of NH3 to ammonium sulfate to be considered irreversible (Nemitz et al., 2004). For ammonium nitrate, both the condensation of NH3 and the evaporation of ammonium nitrate, i.e., gas-particle interconversion, can occur (Nemitz and Sutton, 2004; Nemitz et al., 2004; Pryor et al., 2001; van Oss et al., 1998).
Atmospheric deposition is an important process in terms of the nitrogen input to a forest, although excessive loads may have harmful effects. Atmospheric deposition is divided into dry and wet deposition. For ammoniacal nitrogen, dry deposition of NH3 and pNH4 (collectively NHx) occurs in addition to wet deposition of ammonium ions. However, the atmosphere-forest exchange of NHx is bidirectional. A forest can be an emitter of NH3 through stomatal emissions, re-evaporation of NHx that has deposited onto leaf surfaces (cuticular emission), and soil emissions (Neirynck and Ceulemans, 2008; Andersen et al., 1999; Wyers and Erisman, 1998; Langford and Fehsenfeld, 1992). Condensation of NH3 near the canopy increases and decreases the air concentrations of pNH4 and NH3, respectively, which may cause an apparent emission and deposition of pNH4 and NH3, respectively; the opposite is true for the evaporation of pNH4. Therefore, in terms of the atmosphere-forest exchange of NHx, dry deposition of NHx to the forest, NH3 emissions from the forest, and gas-particle interconversion near the forest canopy are important processes for its quantitative evaluation.
A number of studies regarding the atmosphere-forest exchange of NHx have been conducted in North America and Europe. By contrast, little information is available pertaining to East Asia, including Japan, except for several field studies (e.g., Hayashi et al., 2009a). The project of Impacts of Aerosols in East Asia on Plants and Human Health (ASEPH) was launched in 2008. The purpose of the present study, as a part of ASEPH activities, was to investigate the diurnal variations in air concentrations and exchange fluxes of NHx over a subalpine deciduous forest in central Japan in summer, when biological activities are at their maximum, as a first step towards accumulating data on the atmosphere-forest exchange of NHx in East Asia.
2. MATERIALS AND METHODS
2. 1 Site Description
The study site (36°24′N, 138°35′E, 1,380 m A.S.L.) was located in a subalpine area, i.e., a cool-temperate deciduous forest on the eastern foot of Mt. Asama, Nagano Prefecture, in central Japan. The forest, with a canopy height of approximately 20 m, stands on nearly flat terrain with a gentle slope of 3° over a distance of more than 600 m in the main upwind direction. The forest is composed mainly of birch (Betula ermanii); other tree species include alder (Alnus hirsuta).
Mt. Asama is an active volcano that emits volcanic gas containing sulfur dioxide (SO2). The distance from the crater to the study site is approximately 5 km. There has been no visible damage to the forest at the study site despite occasional exposure to SO2 from the volcano. The net ecosystem exchange (NEE) of carbon dioxide for the forest at the study site (-682 g CO2 m-2 yr-1) (Nakaya, 2008) is on par with the NEE of a similar remote forest of 40-year-old birch in central Japan that is not influenced by volcanic gas (-598 g CO2 m-2 yr-1) (Saigusa et al., 2002).
2. 2 Instruments
The study site had twin walk-up towers with a height of 28 m from the ground surface. Measurements of the air concentrations were conducted using one tower in the summer from 2 to 8 July 2009. The air concentrations of gases, including NH3, were measured using a filter pack method (EMEP, 2001) with filter holders (NL-O, NILU). A filter pack consisted of five filter holders (Hayashi et al., 2007), in which phosphoric acid impregnated filters were used to collect NH3. The flow rate was 10 L min-1. The air concentrations of particles, including pNH4, were measured using aerosol samplers (MCI sampler, Tokyo Dylec Corporation) with a PM2.5 impactor that separated aerosols into coarse particles and PM2.5 (Matsuda et al., 2010), in which glass fiber filters were used to collect particles. The flow rate was 20 L min-1. The air concentrations were measured at three heights (21, 24, and 27m from the ground surface). Although the filter pack method has some possible artifacts when determining air concentrations, as reported in Section 2.4, the use of as simple a method as possible is advantageous to encourage future relevant studies in East Asia.
Samplers were replaced at 6:00, 10:00, 14:00, and 18:00; the sampling frequency was thrice during the daytime and once at night. Each of the collected sample filters was sealed as soon as possible in a clean polypropylene test tube with a cap to avoid contamination. The target substances collected on the filter were extracted by ultrasonic extraction with 10 mL of deionized water and then analyzed using ion chromatography.
A meteorological observation was conducted on the other tower at a height of 28 m from the ground surface with an ultrasonic anemometer-thermometer (METEK, USA-1) and other meteorological instruments, such as temperature and humidity sensors. Thirty-minute means were recorded to a data logger.
2. 3 Calculation of the Exchange Flux
A gradient method (Erisman and Draaijers, 1995) was applied to determine the exchange flux, F (nmol m-2 s-1). The original equation was modified to calculate the flux as a product of the diffusion velocity, D (m s-1) and the difference in concentration between the two heights, Δc (nmol m-3),
| (1) |
where u* and c* are the friction velocity (m s-1) and the eddy concentration (c*≡{κ (z-d)/φh}(∂c/∂z); nmol m-3), respectively. The terms κ, z, d, φh, and c are the von Karman’s constant (=0.4), the target height (m), the zero plane displacement (m), the stability correction function for heat, and the air concentration (nmol m-3). D is expressed by,
| (2) |
where Ψh is the integrated stability correction function for heat (Erisman and Draaijers, 1995). The term ζ is the Monin-Obukhov stability parameter defined as ζ= (z-d)L-1, where L is the Monin-Obukhov length (m). The subscripts 1 and 2 denote each height (z2>z1). The value of d was expressed as 80% of the canopy height (=16 m) (Matsuda et al., 2010).
The exchange fluxes between the heights of 21 and 27 m were determined. The highest and lowest heights were chosen to maximize the difference in concentration. D was calculated using the 30-minute means of u* and L, and averaged at 4-hour intervals. The exchange flux as a 4-hour mean was then calculated by Eq. (1). The air concentration was assumed to be constant during each sampling period. Although a 12-hour sampling period occurred at night, the exchange flux in the nighttime was also calculated every 4 hours based on the assumption that c was constant over the nighttime.
2. 4 Uncertainty
The filter pack method has some possible artifacts, e.g., the re-evaporation of particles from the upstream stage filter following a temperature rise (Keck and Wittmaack, 2005; Anlauf et al., 1985). However, Sickles et al. (1999) observed good agreement between results from the filter pack method and those of the annular denuder method for measuring pNH4. Daynight separation was applied to the present study for sampling, which was effective in reducing the artifact via the re-evaporation of particles due to large changes in temperature between daytime and nighttime. The mean coefficients of variation of air concentrations determined by the filter pack method were 7.1% and 15.1% for NH3 and pNH4, respectively (Hayashi et al., 2009b), which were taken into account as the error of the filter pack method in the present study.
The phosphoric acid impregnated filters used to collect NH3 had a blank value of 0.13±0.06 μg N as ammonium per sheet with a diameter of 47 mm, which accounted for 4.2-8.7% of the total amount of NH3 per sheet sampled in the present study. The glass fiber filters used for collecting coarse particles had a blank value of 0.28±0.05 μg N as ammonium, and those used for PM2.5 had a blank of 0.13±0.01 μg N, which accounted for around 100% of the total amount of pNH4 per sheet sampled when the air concentrations of particles were extremely low. The detection limit of ion chromatography, 10 μg L-1 as ammonium ion, was sufficiently small to determine the air concentrations of NHx, which corresponded to 0.03 μg N m-3;.
Our previous report (Matsuda et al., 2010) pointed out how little error is induced by calculating the exchange fluxes in the nighttime every 4 hours using the 12-hour mean of the air concentration. This lack of error is attributable to the near-zero concentration differences over time in the nighttime, as described later, which is attributable to the remarkable decreases in air concentrations in the nighttime (Fig. 2). For reference, the absolute value of Δc is smaller than c. Therefore, the uncertainty of Δc seems larger than that of c particularly in the nighttime, when Δc is near zero.
Assuming a 10% error in meteorological observations, the maximum relative errors in D were approximately 20% and 100% in the daytime and nighttime, respectively (Miyata, 2001). The gradient method based on the Monin-Obukhov theory is valid within the inertial sub-layer (Businger, 1986). Therefore, Eq. (2) might underestimate D when applied to the roughness sub-layer, e.g., near the canopy, due to an increase in eddy diffusivity within this layer (Matsuda et al., 2010). Wyers and Duyzer (1997) introduced heightdependent correction factors for the use of the gradient method within the roughness sub-layer, although this correction was not used in the present study because of differences in site conditions.
The measurement error of the filter pack method, the measurement-oriented error in D, and the standard deviation of D for 4-hour mean were used to calculate the errors of the exchange fluxes in the present study.
3. RESULTS AND DISCUSSION
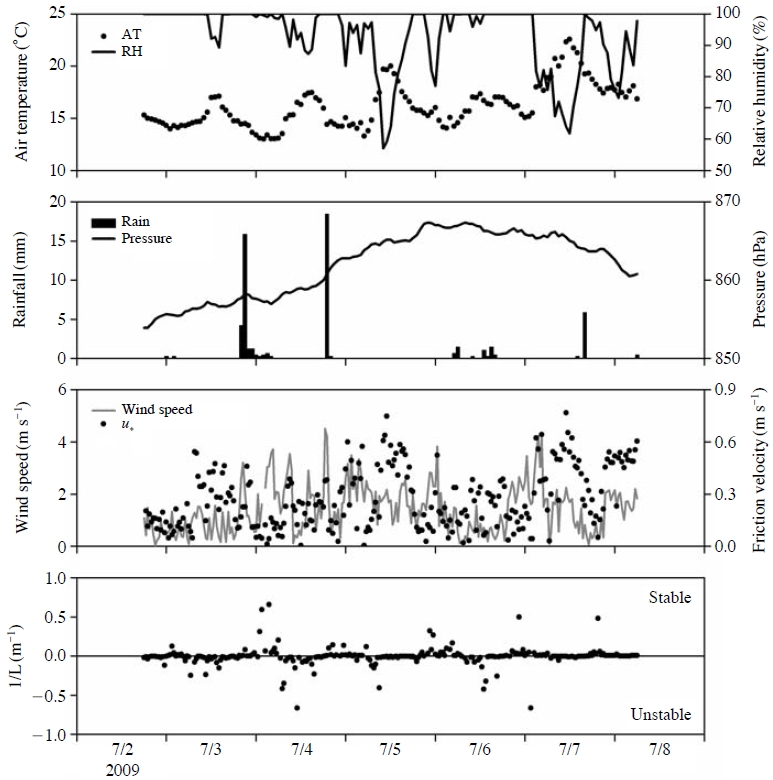
3. 1 Meteorological Conditions
Fig. 1 shows the meteorological variables. The weather at the study site during the research period was generally cloudy, cool, and humid with occasional drizzle or showers. The mean values of air temperature were 17.3 and 15.4°C, in the daytime and nighttime, respectively, and the mean values of relative humidity were 86 and 91% (Matsuda et al., 2010). The high relative humidity throughout the whole day made the canopy surface wet, particularly at night. The weather at the study site was clear in the daytime on 5 July 2009 and in the morning on 7 July 2009, when remarkable increases and decreases in the air temperature and relative humidity, respectively, were observed; u* also had relatively high values during these periods (Fig. 1).
3. 2 Air Concentrations
The air concentrations are expressed as μg N m-3 at standard temperature and pressure (20°C, 1,013 hPa) in the present study. One μg N m-3 of NH3 or pNH4 equals 1.21 or 1.29 μg m-3 of each component, respectively.
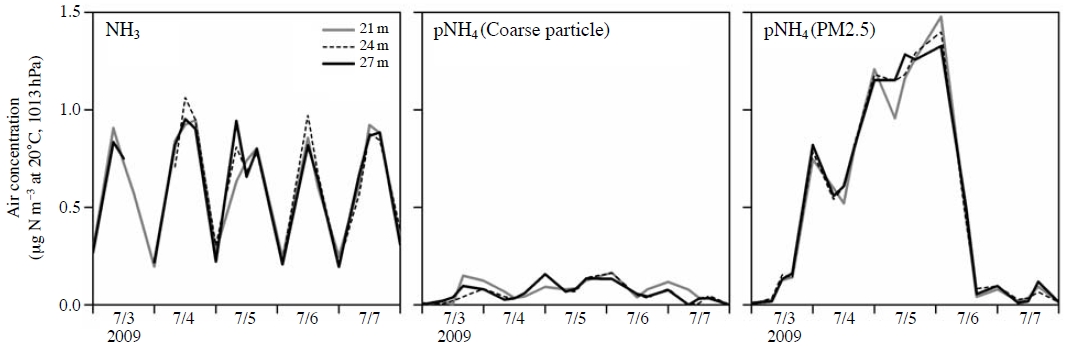
Fig. 2 shows the air concentrations of each component at three heights. The range of NH3 concentrations was 0.20-1.1 μg N m-3. The NH3 concentrations had a circadian variation with a nearly constant amplitude, i.e., high in the daytime and low at night. The concentrations of pNH4 as coarse particles were very low, less than 0.2 μg N m-3. Coarse particles are, therefore, excluded from the following discussion. The concentrations of PM2.5 ammonium were relatively high; the maximum concentration was 1.5 μg N m-3. An obvious feature of the pattern in PM2.5 ammonium concentrations was the lack of any circadian variation, especially in contrast to the clear circadian variation of NH3 (Fig. 2). The PM2.5 ammonium concentration seemed to have a longer-term variation, which might be related to the long-range transport of PM2.5.
Table 1 summarizes the air concentrations at each height. The daytime and nighttime means of the NH3 concentrations (±standard deviation, 27 m height) were 0.77±0.20 and 0.24±0.05 μg N m-3, respectively. The daily mean NH3 concentration at the study site (0.50 μg N m-3, 27 m height) was much lower than those found in some forests affected by NH3-rich air, e.g., the 2-year mean at a Douglas fir forest in The Netherlands (4.3 μg N m-3; Wyers and Erisman, 1998) and the summer-mean at a montane-subalpine forest in Colorado, USA (3.4 μg N m-3; Langford and Fehsenfeld, 1992), whereas it was similar to those of some rural forests, e.g., two field campaigns conducted in spring at a secondary broadleaf forest occasionally affected by animal husbandry activities in Indiana, USA (0.5-1.0 μg N m-3; Pryor et al., 2001), the 5-year mean at a Norway spruce forest surrounded by agricultural lands in western Jutland, Denmark (0.66 μg N m-3; Andersen et al., 1999), and the 3-year mean at a Norway spruce forest in a forested rural area in Saxony, Germany (0.44 μg N m-3; Zimmermann et al., 2006). However, the daily mean NH3 concentration was higher than that in fairly remote forests, e.g., the 2-year mean of a snowless season at a young larch forest in northernmost Hokkaido, Japan (0.31 μg N m-3, 30 m height; Hayashi et al., 2009a) and the summer-mean at a remote hardwood forest in central USA (0.17 μg N m-3; Langford et al., 1992). The site of the present study had the characteristics of a forested rural area with respect to its summer-mean NH3 concentration, although the site was a subalpine forest affected little by direct human activities.
The daytime and nighttime means of PM2.5 ammonium concentration (27 m height) were 0.53±0.52 and 0.57±0.61 μg N m-3, respectively (Table 1). The daily mean PM2.5 ammonium concentration at the study site (0.55 μg N m-3, 27 m height) was lower than the 3-year mean at a Norway spruce forest in a forested rural area in Saxony, Germany (1.2 μg N m-3; Zimmermann et al., 2006), but it was higher than the 2-year mean of a snowless season at a young larch forest in northernmost Hokkaido, Japan (0.29 μg N m-3, 30 m height; Hayashi et al., 2009a).
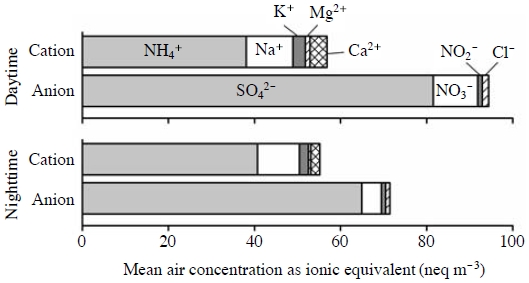
Fig. 3 shows the mean ionic compositions of PM2.5 (27 m height); the compositions were similar regardless of the measurement height (data not shown). The major components were ammonium and sulfate for cations and anions, respectively. The very high correlation between the PM2.5 ammonium and PM2.5 sulfate concentrations (R=0.957, 27 m height, n=20) suggests that PM2.5 ammonium condensed mainly with PM2.5 sulfate. The equivalent sums of anions exceeded those of cations in both the daytime and the nighttime (Fig. 3). Most of the difference between anions and cations probably corresponds to protons, which indicates that PM2.5 was acidic and that ammonium bisulfate was a major component of PM2.5 ammonium as well as ammonium sulfate.
3. 3 Exchange Fluxes
Fig. 4 shows Δc (before correction by the actual air temperature and air pressure), D, and the exchange flux between the heights of 21 and 27 m. Positive and negative values of exchange flux denote downward and upward fluxes, respectively.
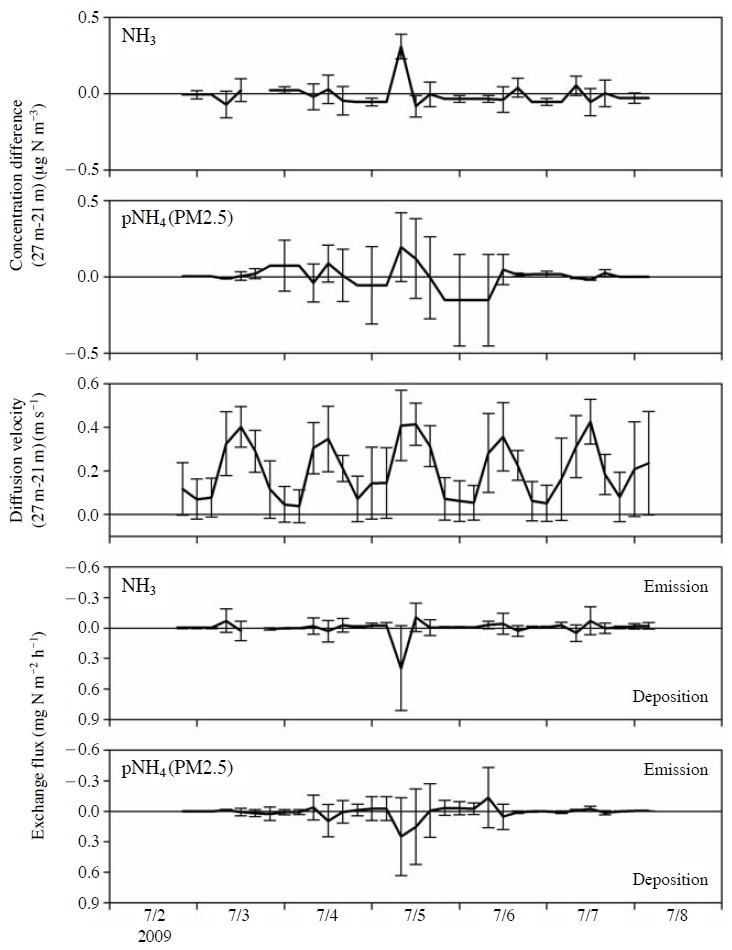
Concentration differences, diffusion velocities, and exchange fluxes of NHx between the heights of 21 and 27 m from the ground surface. Vertical bars denote standard deviations.
The exchange fluxes of NHx were bidirectional (Fig. 4), although the derived fluxes had a certain amount of uncertainty (Section 2.4). Both the maximum and minimum values occurred in the daytime, i.e., 0.39 mg N m-2 hr-1 of downward flux and 0.11 mg N m-2 hr-1 of upward flux for NH3 and 0.25 mg N m-2 hr-1 of downward flux and 0.13 mg N m-2 hr-1 of upward flux for PM2.5 ammonium. The exchange fluxes of NHx in the nighttime were relatively small (Fig. 4), with a range between 0.01 mg N m-2 hr-1 of downward flux and 0.03 mg N m-2 hr-1 of upward flux for NH3 and between 0.03 mg N m-2 hr-1 of downward flux and 0.03 mg N m-2 hr-1 of upward flux for PM2.5 ammonium. The small fluxes at night resulted from the decreases in both Δc and D (Fig. 4). The exchange fluxes of NHx at night can be considered as zero, with a certain degree of uncertainty because of their very small magnitude.
Both NH3 and PM2.5 ammonium had remarkably large downward fluxes on the morning of 5 July 2009, i.e., 0.39 and 0.25 mg N m-2 hr-1, respectively. These large downward fluxes originated primarily from a large Δc (Fig. 4) and, secondarily, from a large D (Fig. 4) induced by a large u* (Fig. 1). For reference, SO2 had a similarly large Δc, but sulfate had a near-zero Δc (data not shown). The deposition velocity (Vd) as a net value was calculated by dividing the exchange flux by the air concentration at a height of 27 m. The large downward fluxes corresponded to remarkably large Vd values of 13.5 and 6.9 cm s-1 for NH3 and PM2.5 ammonium, respectively. For reference, Wyers et al. (1992) and Zimmerman et al. (2006) reported similar mean Vd values of NH3 for forests, i.e., 3.2 and 3.33 cm s-1, respectively. It appears that the showers during the night of 3 to 4 July 2009 (Fig. 1) thoroughly washed and wetted the forest canopy and ground surface, and the subsequent clear sky on the morning of 5 July 2009, which is indirectly shown in the steep decrease in relative humidity (Fig. 1), stimulated convection. Both are necessary conditions to accelerate the dry deposition of NH3, particularly, surface deposition on not only the leaf surface but also other tree bodies such as branches and bark. In addition, the arithmetic mean of Vd during the research period was almost zero for both NH3 and PM2.5 ammonium. Therefore, the NHx exchange between the study forest and the atmosphere seems to be balanced.
Wyers and Erisman (1998) have reported the reevaporation of NH3 from NHx previously deposited on the leaf surface; however, only a weak upward flux of NH3 following the remarkably large deposition of NHx on 5 July 2009 was found in the present study (Fig. 4). The drizzle on 6 July 2009 might have rinsed off the deposited NHx on the leaf surface, effectively reducing the re-evaporation. Thus, the cleaning effect as well as the surface wetness by rainfall should be monitored to evaluate and generalize the gas and particle exchange at a forest.
With respect to the large Vd of PM2.5 ammonium, a Vd of aerosols based on a field measurement at a forest tends to be larger than a Vd derived from a theoretical model (e.g., Matsuda et al., 2010; Horváth, 2003; Garland, 2001; Erisman et al., 1997). Furthermore, the clear decrease in the air concentration of NH3 near the canopy on the morning of 5 July 2009 (Fig. 2) was probably due to enhanced dry deposition, which might induce the evaporation of PM2.5 ammonium via a particle-to-gas conversion to compensate for the decreased NH3 concentration. The simultaneous decrease in the air concentration of PM2.5 ammonium near the canopy (Fig. 2) supports this interpretation, in which the large downward flux of PM2.5 ammonium should be attributable to the apparent emission of NH3 (Section 3.4) to a certain extent.
However, ammonium sulfate and ammonium bisulfate, i.e., the most dominant salts in the present study (Fig. 3), have low vapor pressures. Therefore, these particles were unlikely to contribute to any particle-to-gas conversion. Meanwhile, both nitrate and chloride had very low concentrations (Fig. 3), and they were also unlikely to be a main source of any particleto- gas conversion. The counter anion of the PM2.5 ammonium that vaporized was unidentified.
The causes of the upward flux of NH3 occasionally found in the daytime (Fig. 4) are divided into direct emissions from the study forest and apparent emissions due to particle-to-gas conversion (Section 3.4). By contrast, the cause of the upward flux of PM2.5 ammonium should be gas-to-particle conversion (Section 3.4) because PM2.5 is mainly secondary particles and direct emissions from a forest are unlikely to occur.
3. 4 NH3 Emission from and Deposition to the Forest
A forest can emit NH3 from stomata, leaf surfaces, and soil surfaces (Hayashi et al., 2009a; Neirynck and Ceulemans, 2008; Andersen et al., 1999; Wyers and Erisman, 1998; Langford and Fehsenfeld, 1992). A forest in which trees and the understory are cut can also emit NH3 as a result of the decomposition of fresh organic matter (Hayashi et al., 2009a) in addition to volatilization after nitrogen fertilization. However, the study forest was a natural forest and thus not under management. Hence, the possible causes of NH3 emission from the study forest are limited to stomatal, leaf surface, and/or soil surface emissions.
Stomatal NH3 emission from a forest has been reported (Neirynck and Ceulemans, 2008; Andersen et al., 1999; Wyers and Erisman, 1998; Langford and Fehsenfeld, 1992). Stomatal NH3 emission is, however, limited to the daytime because of stomatal closure at night. Langford and Fehsenfeld (1992) reported an average value of the compensation point of 0.8 ppb (=0.48 μg N m-3) for a remote natural forest in the central USA. All air concentrations of NH3 in the daytime in the present study exceeded this value (Fig. 2), which suggests that stomatal NH3 emissions were not likely to occur in the study forest. However, the difference in tree species between Japan and the USA should be noted.
Leaf-surface NH3 emissions might occur in a forest (Neirynck and Ceulemans, 2008; Wyers and Erisman, 1998) when the NH3 originates from NHx that previously deposited on the leaf surface. The accumulated NH3 might be reemitted to the atmosphere in conjunction with a subsequent dry period, which is the daytime in many cases (Wyers and Erisman, 1998). Leaf-surface emissions, including emissions from other tree bodies, might contribute to the emission of NH3 following the remarkably large deposition of NH3 on the morning of 5 July, 2009 (Fig. 4), in addition to the apparent emission as described later. The relatively low air concentrations of NH3 (Fig. 2, Table 1) were advantageous to inducing leaf-surface NH3 emissions.
In terms of soil-surface NH3 emissions, the pH values of rainwater and the soil solution at a depth of 10 cm at the study site were 4-5 and less than 6, respectively (Ikeda et al., 2004). Soil-surface NH3 emissions are unlikely to be significant under such acidic conditions (Hayashi et al., 2008).
As described above, leaf-surface NH3 emission was the only possible direct emission process of NH3 in the study forest, other than the apparent emission due to gas-particle interconversion discussed below.
When a forest emits NH3, the emitted NH3 encounters acidic substances, e.g., sulfuric acid and nitric acid, in the atmosphere near the canopy and can condense with acidic substances to form PM2.5 ammonium. This process results in the apparent emission of PM2.5 ammonium, which simultaneously and apparently decreases the emission flux of NH3. The upward flux of PM2.5 ammonium occasionally shown in the daytime (Fig. 4) was perhaps attributable to this apparent emission.
Meanwhile, evaporable ammonium salts, such as ammonium nitrate, release NH3 due to their evaporation in the atmosphere with a temperature rise (e.g., Pryor et al., 2001). Evaporation near the canopy is likely to occur in the daytime in good weather because of the warming effect of solar radiation on the canopy. In this case, the tendency for deposition of PM2.5 is enhanced but that of NH3 is weakened and even turns into an emission tendency in some cases. Furthermore, a portion of ammonium salts might evaporate to compensate for the decrease in the air concentration of NH3 when a large amount of NH3 deposition occurs (Section 3.3). However, the evaporation of ammonium salts does not seem to be very strong in the study forest because the main component of PM2.5 was ammonium sulfate (Fig. 3), which has a low vapor pressure.
4. CONCLUSIONS
The air concentrations and exchange fluxes of NHx were investigated at a subalpine deciduous forest in central Japan during a summer week. The NH3 concentrations (daily mean of 0.50 μg N m-3) showed a clear circadian variation with a nearly constant amplitude, high in the daytime (0.77 μg N m-3 on average) and low at night (0.24 μg N m-3 on average). The concentrations of pNH4 for coarse fractions were very low (0.07 μg N m-3 as the daily mean). By contrast, those for PM2.5 ammonium were relatively high (0.55 μg N m-3 as the daily mean), and the concentration pattern of PM2.5 ammonium had a longer cycle than a circadian variation, which was remarkably similar to that of PM2.5 sulfate and might be related to the longrange transport of PM2.5. In comparison with the early studies done in North America and Europe, the study site had features characteristic of a forested rural area with respect to the summer-mean NH3 concentration. The PM2.5 at the study site was composed mainly of ammonium ions and sulfate ions. The PM2.5 was generally acidic.
The exchange fluxes of NHx in the daytime were bidirectional, but those at night could be considered as zero because of their very small magnitude. However, for PM2.5 ammonium in particular, changes in the status of long-range transportation might affect the air concentrations and the exchange fluxes at the study forest. Both NH3 and PM2.5 ammonium had remarkably large downward fluxes on the morning of 5 July 2009, i.e., 0.39 and 0.25 mg N m-2 hr-1, respectively, which corresponded to Vd values of 13.5 and 6.9 cm s-1, respectively. The large Vd is probably attributable to the preceding rainfall that washed and wetted the forest canopy and the strong convection in the morning. However, the mean Vd during the research period was almost zero for both NH3 and PM2.5 ammonium. The atmosphere-forest exchange of NHx at the study forest is likely balanced.
The possible cause of the upward fluxes of NH3 occasionally found at the study forest was limited to leaf-surface emissions. The air concentrations of NH3 in the daytime most likely exceed the compensation point of NH3, resulting in dry deposition through stomata instead of stomatal emission. The relatively low pH value of the soil solution at the study forest should inhibit soil emissions of NH3 as well.
In terms of the gas-particle interconversion at the study forest, which induces an apparent emission/deposition of NHx, the upward fluxes of PM2.5 ammonium occasionally found in the daytime might be attributable to the apparent emission of PM2.5 ammonium due to the condensation of NH3 emitted from the canopy. Meanwhile, the contribution of the evaporation of ammonium salts, which results in an apparent deposition of PM2.5 ammonium, is unlikely to be important at the study forest because the PM2.5 was composed mainly of ammonium sulfate, which has a low vapor pressure.
Acknowledgments
We would like to express our gratitude to Prof. Shiro Hatakeyama and Prof. Takeshi Izuta, Tokyo University of Agriculture and Technology, Japan, for their support of the present study. The authors also thank Mr. Yoshifumi Fujimura, Mr. Takashi Miyake, and Mr. Masayuki Miyanaga, Meisei University, Japan, for their assistance with measurements and chemical analyses. This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas, No. 20120012, provided by the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
REFERENCES
- Andersen, H.V., Hovmand, M.F., Hummelshoj, P., Jensen, N.O., (1999), Measurements of ammonia concentrations, fluxes and dry deposition velocities to a spruce forest 1991-1995, Atmospheric Environment, 33, p1367-1383.
-
Anlauf, K.G., Fellin, P., Wiebe, H.A., Schiff, H.I., Mackay, G.I., Braman, R.S., Gilbert, R., (1985), A comparison of three methods for measurement of atmospheric nitric acid and aerosol nitrate and ammonium, Atmospheric Environment, 19, p325-333.
[https://doi.org/10.1016/0004-6981(85)90100-3]

-
Bardouki, H., Liakakou, H., Economou, C., Sciare, J., Smolík, J., Ždímal, V., Eleftheriadis, K., Lazaridis, M., Dye, C., Mihalopoulos, N., (2003), Chemical composition of size-resolved atmospheric aerosols in the eastern Mediterranean during summer and winter, Atmospheric Environment, 37, p195-208.
[https://doi.org/10.1016/s1352-2310(02)00859-2]

- Bouwman, A.F., van Vuuren, D.P., Derwent, R.G., Posch, M., (2002), A global analysis of acidification and eutrophication of terrestrial ecosystems, Water, Air, and Soil Pollution, 141, p349-382.
-
Businger, J.A., (1986), Evaluation of the accuracy with which dry deposition can be measured with current micrometeorological techniques, Journal of Climate and Applied Meteorology, 25, p1100-1124.
[https://doi.org/10.1175/1520-0450(1986)025<1100:eotaww>2.0.co;2]

- EMEP, (2001), EMEP manual for sampling and chemical analysis, EMEP/CCC-Report 1/95, Available from: http://www.emep.int/.
-
Erisman, J.W., Draaijers, G.P.J., (1995), Atmospheric deposition in relation to acidification and eutrophication, Studies in Environmental Science 63, Elsevier, p55-75.
[https://doi.org/10.1016/s0166-1116(06)x8328-0]

- Erisman, J.W., Draaijers, G., Duyzer, J., Hofshreuder, P., van Leeuwen, N.F.M., Römer, F., Ruijgrok, W., Wyers, P., Gallagher, M., (1997), Particle deposition to forestssummary of results and application, Atmospheric Environment, 31, p321-332.
-
Erisman, J.W., Schaap, M., (2004), The need for ammonia abatement with respect to secondary PM reductions in Europe, Environmental Pollution, 129, p159-163.
[https://doi.org/10.1016/j.envpol.2003.08.042]

- Galloway, J.N., Dentener, F.J., Capone, D.G., Boyer, E.W., Howarth, R.W., Seitzinger, S.P., Asner, G.P., Cleveland, C.C., Green, P.A., Holland, E.A., Karl, D.M., Michaels, A.F., Porter, J.H., Townsend, A.R., Vörösmarty, C.J., (2004), Nitrogen cycles: past, present, and future, Biogeochemistry, 70, p153-226.
- Garland, J.A., (2001), On the size dependence of particulate deposition, Water, Air and Soil Pollution: Focus, 1, p323-332.
-
Hayashi, K., Komada, M., Miyata, A., (2007), Atmospheric deposition of reactive nitrogen on turf grassland in central Japan: Comparison of the contribution of wet and dry deposition, Water, Air, and Soil Pollution: Focus, 7, p119-129.
[https://doi.org/10.1007/s11267-006-9096-4]

- Hayashi, K., Koga, N., Yanai, Y., (2009b), Effects of fieldapplied composted cattle manure and chemical fertilizer on ammonia and particulate ammonium exchanges at an upland field, Atmospheric Environment, 43, p5702-5707.
-
Hayashi, K., Nishimura, S., Yagi, K., (2008), Ammonia volatilization from a paddy field following applications of urea: Rice plants are both an absorber and an emitter for atmospheric ammonia, Science of the Total Environment, 390, p485-494.
[https://doi.org/10.1016/j.scitotenv.2007.10.037]

- Hayashi, K., Takagi, K., Noguchi, I., Fukuzawa, K., Takahashi, H., Fukazawa, T., Shibata, H., Fujinuma, Y., (2009a), Ammoniacal nitrogen emission from a young larch ecosystem afforested after clear-cutting of a pristine forest in northernmost Japan, Water, Air, and Soil Pollution, 200, p33-46.
-
Hayashi, K., Yan, X.Y., (2010), Airborne nitrogen load in Japanese and Chinese agroecosystems, Soil Science and Plant Nutrition, 56, p2-18.
[https://doi.org/10.1111/j.1747-0765.2009.00423.x]

-
Horváth, L., (2003), Dry deposition velocity of PM2.5 ammonium sulfate particles to a Norway spruce forest on the basis of S- and N-balance estimations, Atmospheric Environment, 37, p4419-4424.
[https://doi.org/10.1016/s1352-2310(03)00584-3]

- Ikeda, H., Yasuike, S., Kobayashi, T., Nakaya, K., Suzuki, C., (2004), Carbon stock and chemistry in deciduous forest-soil system, CRIEPI Research Report, Central Research Institute of Electric Power Industry, Japan, U03069 p19, (in Japanese with English abstract).
- IPCC, (2007), The Physical Science Basis, Contribution of Working Group I to the Fourth Assessment Report of Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge.
-
Keck, L., Wittmaack, K., (2005), Effect of filter type and temperature on volatilization losses from ammonium salts in aerosol matter, Atmospheric Environment, 39, p4093-4100.
[https://doi.org/10.1016/j.atmosenv.2005.03.029]

- Langford, A.O., Fehsenfeld, F.C., (1992), Natural vegetation as a source or sink for atmospheric ammonia: a case study, Science, 25, p581-583.
-
Langford, A.O., Fehsenfeld, F.C., Zachariassen, J., Schimel, D.S., (1992), Gaseous ammonia fluxes and background concentrations in terrestrial ecosystems of the United States, Global Biogeochemical Cycles, 6, p459-483.
[https://doi.org/10.1029/92gb02123]

-
Matson, P., Lohse, K.A., Hall, S.J., (2002), The globalization of nitrogen deposition: Consequences for terrestrial ecosystems, Ambio, 31, p113-119.
[https://doi.org/10.1639/0044-7447(2002)031[0113:tgondc]2.0.co;2]

-
Matsuda, K., Fujimura, Y., Hayashi, K., Takahashi, A., Nakaya, K., (2010), Deposition velocity of PM2.5 sulfate in the summer above a deciduous forest in central Japan, Atmospheric Environment, 44, p4582-4587.
[https://doi.org/10.1016/j.atmosenv.2010.08.015]

- Miyata, A., (2001), Observational study on methane exchange between wetland ecosystems and the atmosphere, Bulletin of National Institute for Agro-Environmental Sciences, 19, p61-183, Available from: http://www.niaes.affrc.go.jp/index_e.html.
- Nakaya, K., (2008), Study of improvement on evaluation method of energy and mass exchange between atmosphere and forest ecosystem, Memories of the Research Faculty of Agriculture, Hokkaido University, 29, p149-213, (in Japanese with English abstract).
-
Neirynck, J., Ceulemans, R., (2008), Bidirectional ammonia exchange above a mixed coniferous forest, Environmental Pollution, 154, p424-438.
[https://doi.org/10.1016/j.envpol.2007.11.030]

- Nemitz, E., Sutton, M.A., (2004), Gas-particle interactions above a Dutch heathland: III. Modelling the influence of the NH3-HNO3-NH4NO3 equilibrium on size-segregated particle fluxes, Atmospheric Chemistry and Physics, 4, p1025-1045.
-
Nemitz, E., Sutton, M.A., Wyers, G.P., Otjes, R.P., Mennen, M.G., van Putten, E.M., Gallagher, M.W., (2004), Gas-particle interactions above a Dutch heathland: II. Concentrations and surface exchange fluxes of atmospheric particles, Atmospheric Chemistry and Physics, 4, p1007-1024.
[https://doi.org/10.5194/acpd-4-1519-2004]

- Neuberger, M., Schimek, Jr., M.G., Horak, F., Moshammer, H., Kundi, M., Frischer, T., Gomiscek, B., Puxbaum, H., Hauck, H., AUPHEP-Team, (2004), Acute effects of particulate matter on respiratory diseases, symptoms and functions: epidemiological results of the Austrian project on health effects of particulate matter (AUPHEP), Atmospheric Environment, 38, p3971-3981.
-
Niemi, J.V., Saarikoski, S., Aurela, M., Tervahattu, H., Hillamo, R., Westphal, D.L., Aarnio, P., Koskentalo, T., Makkonen, U., Vehkamaki, H., Kulmala, M., (2009), Long-range transport episodes of fine particles in southern Finland during 1999-2007, Atmospheric Environment, 43, p1255-1264.
[https://doi.org/10.1016/j.atmosenv.2008.11.022]

-
Pryor, S.C., Barthelmie, R.J., Sørensen, L.L., Jensen, B., (2001), Ammonia concentrations and fluxes over a forest in the midwestern USA, Atmospheric Environment, 35, p5645-5656.
[https://doi.org/10.1016/s1352-2310(01)00259-x]

-
Saigusa, N., Yamamoto, S., Murayama, S., Kondo, H., Nishimura, N., (2002), Gross primary production and net ecosystem exchange of a cool-temperate deciduous forest estimated by the eddy covariance method, Agricultural and Forest Meteorology, 112, p203-215.
[https://doi.org/10.1016/s0168-1923(02)00082-5]

- Sickles II, J.E., Hodson, L.L., Vorburger, L.M., (1999), Evaluation of the filter pack for long-duration sampling of ambient air, Atmospheric Environment, 33, p2187-2202.
-
van Oss, R., Duyzer, J.H., Wyers, P., (1998), The influence of gas-to-particle conversion on measurements of ammonia exchange over forest, Atmospheric Environment, 32, p465-471.
[https://doi.org/10.1016/s1352-2310(97)00280-x]

-
Wyers, G.P., Duyzer, J.H., (1997), Micrometeorological measurement of the dry deposition flux of sulphate and nitrate aerosols to coniferous forest, Atmospheric Environment, 31, p333-343.
[https://doi.org/10.1016/s1352-2310(96)00188-4]

- Wyers, G.P., Erisman, J.W., (1998), Ammonia exchange over coniferous forest, Atmospheric Environment, 32, p441-451.
-
Wyers, G.P., Vermeulen, A.T., Slanina, J., (1992), Measurement of dry deposition of ammonia on a forest, Environmental Pollution, 75, p25-28.
[https://doi.org/10.1016/0269-7491(92)90052-c]

-
Zimmermann, F., Plessow, K., Queck, R., Bernhofer, C., Matschullat, J., (2006), Atmospheric N- and S-fluxes to a spruce forest: Comparison of inferential modelling and the throughfall method, Atmospheric Environment, 40, p4782-4796.
[https://doi.org/10.1016/j.atmosenv.2006.03.056]