A Detection of Airborne Particles Carrying Viable Bacteria in an Urban Atmosphere of Japan
Abstract
Viable bacteria on water-insoluble airborne particles were detected in the urban atmosphere of Kumamoto (134°45′E, 32°28′N), Japan, in autumn 2008. Airborne particles were collected onto film-covered Cu meshes under clear weather conditions. The samples were stained by fluorescent stains, and then viewed and photographed with an epifluorescent microscope. Non-biological and bacterial parts in particles larger than 0.8 μm were distinguished by their morphologies, fluorescent colors and fluorescent intensities. Bacterial viable statuses were discriminated according to cell membrane damage. In total, 2681 particles were investigated and it was found that 78 airborne particles were associated with bacteria. Viable bacteria were identified on 48 particles. A few particles carried multiple viable bacteria. These results provide the evidence that airborne particles act as carriers of viable bacteria in the atmosphere.
Keywords:
Biological particle, Viable bacteria, Airborne particle, Fluorescent staining, Urban atmosphere1. INTRODUCTION
Biological particles including microbes are ubiquitous in the atmosphere (Jaenicke et al., 2007; Jaenicke, 2005). Meteorological factors such as temperature, humidity, winds and solar radiations induce microbial aerosolization and, as a consequence, these factors typically control microbial abundance and composition in the near surface atmosphere (Burrows et al., 2009; Jones and Harrison, 2004). Airborne microbes can initiate cloud formation by acting as ice nuclei and/or droplet nuclei, and thus indirectly influence the climate (Pratt et al., 2009; Prenni et al., 2009; Möhler et al., 2007; Sun and Ariya, 2006). Studies of airborne microbes are also conducted to estimate their effect on public health (Chi and Li, 2005; Fabian et al., 2005; Griffin and Kellogg, 2004).
Bacteria attached to airborne particles have been traditionally argued, and were frequently detected by indirect methods (e.g. Tong and Lighthart, 2000). So far, the presence of bacteria was rarely observed directly on airborne particles with an efficient method and only a few observation data are available. For example, Casareto et al. (1996) reported that bacteria attached to mineral particles in rain water might be able to play a role in ice nucleation. Studies at the eastern edge of the TaklaMakan desert found the adherence of bacteria to mineral particles in aerosol samples collected at 800-1,200 m above the ground (Yamada et al., 2010; Maki et al., 2008). These results indicate the presence of water-insoluble particles, especially, mineral ones suspended in the air as bacterial carriers.
It has been revealed that viable status of airborne bacteria is crucial to public health and downwind ecosystems (Griffin and Kellogg, 2004; Griffin et al., 2003). Many researchers suggested that bacteria attached to airborne particles might have longer survival time in the atmosphere due to the protection of the particles, and the attachment could enable them to transport in viable state in the air (Lighthart and Tong, 1998; Lighthart and Shaffer, 1997). However, viable bacteria on airborne particles have not been identified and the viable status has not been documented.
In this study, direct observations of viable bacteria on water-insoluble airborne particles in an urban atmosphere in Japan are reported. An epifluorescent microscope coupled with fluorescent stains, which is one of the most common approaches to analyze viable bacteria (Boulos et al., 1999), was employed in the identification of bacteria.
2. EXPERIMENTAL METHOD
Sample collections were conducted on a balcony (about 8 m above the ground) and on the roof (about 25 m above the ground) of a building on the campus of the Prefectural University of Kumamoto, Japan (130°45′E, 32°48′N) in September, November and December, 2008. The collections were carried out when the weather was clear and governed by high pressure systems. Table 1 shows the details of the sample collection dates, periods and the relevant weather conditions. Kumamoto is a city in the center of the island of Kyushu. Kyushu is located in southwestern Japan and its land area composes one of the four major landmasses of Japan (Fig. 1). The ground altitude of the sampling place is 32 m above sea level and approximately 18km west of the sampling site is the Ariake sound. Weather data at the Kumamoto Meteorological Observatory (approximately 5.6 km west of the sampling site) distributed by the Japan Meteorological Agency are applied in this study to show the relevant weather conditions (open site: http://www.jma.go.jp/jma/index.html).

Sampling dates, periods and the relevant weather conditions. The temperature (T), relative humidity (RH) and pressure (P) are the average. Information of temperature, relative humidity and pressure were from Japan Meteorological Agency (open site: http://www.jma.go.jp/jma/index/html).
Particles suspended in the air were collected with a single-stage cascade impactor (Stage 1: PIXE International Corporation, USA) at a flow rate of 1.0 L min-1. Each sample was obtained by trapping airborne particles onto a Cu mesh for 5 minutes. Each mesh was pre-covered with carbon-sprayed dichloroethane film. Given that the density is 1.0 g cm-3, the estimated 50% cut-off aerodynamic diameter of the impactor is 0.3 μm.
The samples were stained by using LIVE/DEAD BacLight Bacterial Viability Kit (BacLight stain; Molecular Probes, Ink., USA, L13152). BacLight stain is composed of two fluorescent stains: SYTO9 and propidium iodide (PI) (Boulos et al., 1999). SYTO9 can label all bacterial cells while PI can only label membranedamaged bacterial cells. As a result, if membrane-damaged bacterial cells appear, they will be stained with both stains and emit red fluorescence. If intact bacterial cells appear, they will be stained with only SYTO9 and emit green fluorescence. In this study, viable and nonviable bacteria were distinguished by cell membrane damage.
Stock solution was preliminary prepared using Bac-Light stain according to the manufacture’s description, and stored at -20°C. For the samples of each day, about 1 mL of staining fluid was prepared by the dilution of 30 μL stock solution into 970 μL sterile particle-free water. The staining was conducted within 3 hours after sample collection. Each sample mesh was stained by the fluid for 15 minutes in dark at room temperature. After that, the mesh was rinsed twice for 5 minutes in sterile particle-free water. Then, the mesh was placed on a slide and covered by a cover glass.
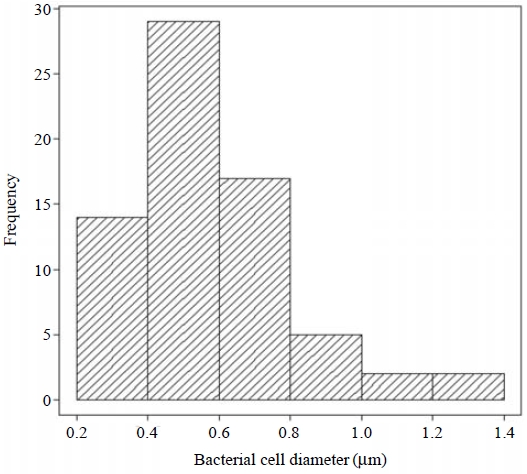
The samples were observed and photographed with an epifluorescent microscope (NIKON ECLIPSE 80i, JAPAN) equipped with a mercury 100 w lamp. Individual particles and bacteria were distinguished by their morphologies, fluorescent colors and fluorescent intensities under the microscope. Observations were conducted under blue excitation rays (excitation filter: 450-490 nm, emission filter: 520 nm) and visible light at 1000 magnification. More than 100 water-insoluble particles were observed in the samples of each day. In the analysis, only particles larger than 0.8 μm were analyzed due to the limited resolution of the microscope. In addition, bacterial cell diameters were calculated as the mean of longest and shortest parts of the bacteria on particles from epifluorescent photographs with an image analyzing software (Lumina Vision, Mitani Corporation, Japan).
3. RESULTS AND DISCUSSION
Fig. 2 shows the pictures of bacterium-carrying particles observed by the epifluorescent microscope. Under the blue excitation rays, a particle emitted yellowish fluorescence (Fig. 2a1). There were two spots emitting strong fluorescence in green color (enlarged parts) on the particle. Under both blue excitation rays and visible light, the particle did not emit fluorescence, and was optically transparent (Fig. 2a2). Since dichloroethane film does not emit any fluorescence, the yellowish part was the water-insoluble body of the particle and the strongly-fluorescing parts on it were viable bacteria, i.e. the particle was a viable-bacterium carrier (Fig. 2a3). Similarly, particles carrying non-viable bacteria were identified by the same procedure using Bac-Light stain and an example is shown in Fig. 2b1 and Fig. 2b2. The bacterium-carrying particles as shown in Fig. 2 were very likely mineral particles in regard to their morphology and size. However, such particles are called water-insoluble particles for the sake of accuracy in the following descriptions because of the limit of the method.
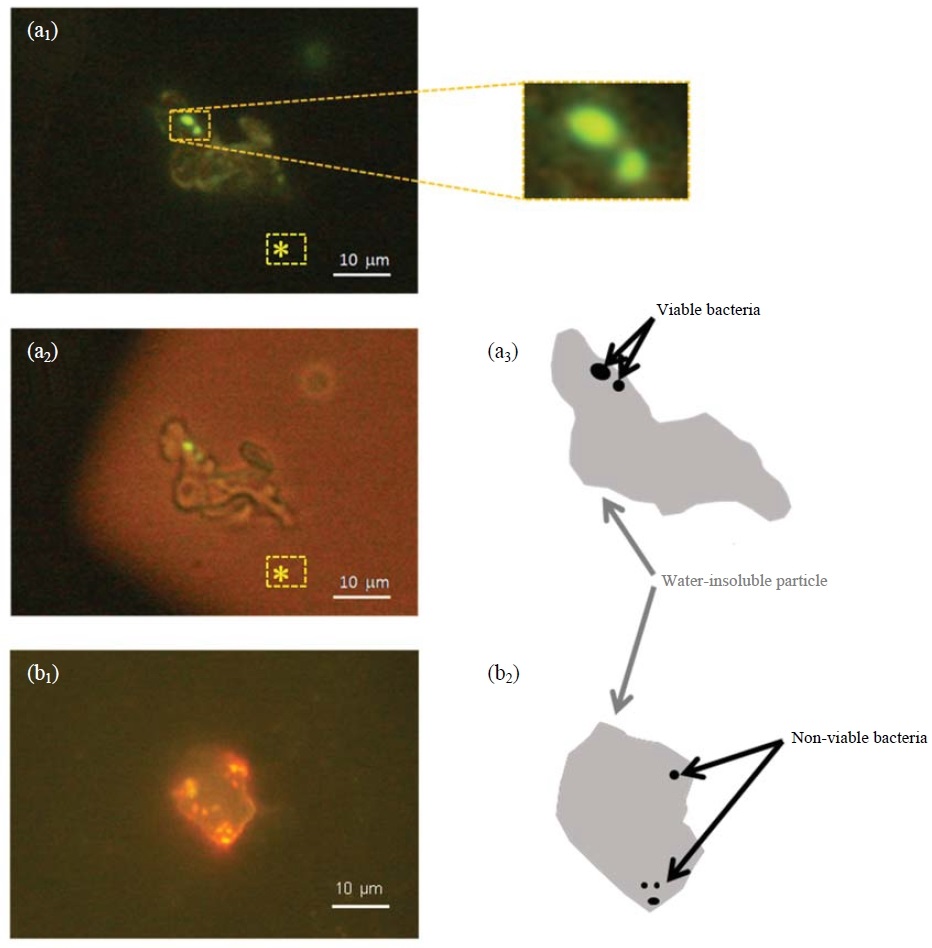
Epifluorescence micrographs of bacterium-carrying particles: (a1) and (a2) show the same particle when irradiated by blue excitation rays and by blue excitation rays and visible light, respectively, and (a3) is the sketched structure of the particle. (b1) shows another particle when irradiated by blue excitation rays and (b2) is its sketched structure. *: parts of dichloroethane film.
In total, 2681 particles larger than 0.8 μm in the 16 samples collected during the entire sampling period were investigated. It was found that 78 particles were bacterium-carrying particles, in which 48 carried viable bacteria (Table 2). A few particles carried multiple bacteria (Fig. 2). The average ratio of bacterium-carrying particles to total water-insoluble particles is 3% with the maximum of 9% on September 8th and the minimum of 0% on September 3rd, indicating that a very small fraction of water-insoluble particles were bacterial carriers. However, the ratios are considered to be the lower bound. The reason is that bacteria originally attached to particles might be separated from the particles during the pretreatments for fluorescent investigation, which could cause the reduction of detected ratios of bacterium-carrying particles.

Observational results of bacteria attached to airborne particles by the epifluorescent microscope with LIVE/DEAD BacLight bacterial viability kit.
It was also confirmed that, all the bacterium-carrying particles were larger than 5.0 μm. This result is similar to that obtained by Maki et al. (2008) at the eastern edge of the TaklaMakan desert by using an epifluorescent microscope with a fluorescent stain. Previous studies have shown that bacteria are usually smaller than 1.0 μm in natural environment (Torrella and Morita, 1981; Bae et al., 1972). It is probable for an airborne particle smaller than 5.0 μm to carry bacteria in the point of view of size. Unfortunately because of the limit of the resolution of optical microscopy, it was unable to determine whether a fluorescing bacterium existed individually or coherently with a particle smaller than 5.0 μm. To the extent of our knowledge, bacterium-carrying particles which are smaller than 5.0μm have rarely been found in previous studies. Because of the small number of fluorescent particles in this range, it is unlikely that particles smaller than 5.0 μm are substantial carriers of bacteria in the air although this is difficult to be clarified with the current techniques at this stage. In this study, only particles clearly identified as shown in Fig. 2 were categorized as bacterium-carrying particles.
Most of the bacteria on particles were smaller than 1.0 μm and they showed or were close to a spherical shape with the mean size of 0.6 μm(Fig. 2 and Fig. 3). If a 0.6μm bacterium has a spherical shape, the surface area of an airborne particle of 5 μm is approximately 70 times larger than that of the bacterium. Although the simplification of spherical shape had large uncertainties, coarse particles in the air have sufficient surface areas to carry multiple bacteria, suggesting that airborne particles could act as transport media to carry multiple viable or non-viable bacteria.
For reference, 104 bacteria were detected on the 78 bacterium-carrying particles in the samples. Among the bacteria, 70 were viable and 34 were non-viable, suggesting approximately two third of bacteria on particles were viable. This is within 57-92%, the reported range of atmospheric viable microbes according to cell membrane damages under various weather conditions (Li and Huang, 2006; Sahu et al., 2005). It is expected that the coherence of bacteria on particles could extend the time of the bacteria in viable state in the air because of reduced exposure directly to the environment stress. In other words, the coherence benefits the dispersion of viable bacteria via the air. Although the data obtained here might have large uncertainties, the results show that some large particles, carrying viable bacteria, suspended in the air. This has been suggested to be an atmospheric process potentially important for public health and downwind ecosystems, and needs to be studied in details.
4. SUMMARY
Viable bacteria on water-insoluble airborne particles were investigated in the urban atmosphere of Kumamoto, Japan. Bacteria attached to airborne particles and their viable activities were confirmed by an epifluorescent microscope using LIVE/DEAD BacLight Bacterial Viability Kit. Among the 2681 investigated particles, 78 particles were associated with bacteria and 48 particles were confirmed as viable-bacterium carriers. According to morphology and size, the particles are believed to be mineral particles. Some particles carried multiple bacteria. Almost all bacteria on particles were smaller than 1 μm diameter, which is consistent with the size of bacteria in natural environment. It is evidenced that airborne particles could be carriers of viable bacteria in the air.
Acknowledgments
This research was funded by the Sasakawa Scientific Research Grant from the Japan Science Society, and partly supported by the JSPS under Grant-in-Aid for Exploratory Research. The authors thank Prof. J. Melton for his assistance in editing the words and grammar of this paper.
REFERENCES
- Bae, H.C., Cota-robles, E.H., Casida, L.E., (1972), Microflora of soil as viewed by transmission electron microscopy, Applied Microbiology, 23, p637-648.
-
Boulos, L., Prévost, M., Barbeau, B., Coallier, J., Desjardins, R., (1999), LIVE/DEAD® BacLightTM: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water, Journal of Microbiological Methods, 37, p77-86.
[https://doi.org/10.1016/s0167-7012(99)00048-2]

-
Burrows, S.M., Elbert, W., Lawrence, M.G., Pöschl, U., (2009), Bacteria in the global atmosphere - part 1: review and synthesis of literature data for different ecosystems, Atmospheric Chemistry and Physics, 9, p9263-9280.
[https://doi.org/10.5194/acp-9-9263-2009]

-
Casareto, B.E., Suzuki, Y., Okada, K., Morita, M., (1996), Biological micro-particles in rain water, Geophysical Research Letters, 23, p173-176.
[https://doi.org/10.1029/95gl03785]

-
Chi, M.-C., Li, C.-S., (2005), Fluorochrome and fluorescent in situ hybridization to monitor bioaerosols in swine buildings, Aerosol Science and Technology, 39, p1101-1110.
[https://doi.org/10.1080/02786820500421539]

-
Fabian, M.P., Miller, S.L., Reponen, T., Hernandez, M.T., (2005), Ambient bioaerosol indices for indoor air quality assessments of flood reclamation, Journal of Aerosol Science, 36, p763-783.
[https://doi.org/10.1016/j.jaerosci.2004.11.018]

-
Griffin, D.W., Kellogg, C.A., Garrison, V.H., Lisle, J.T., Borden, T.C., Shinn, E.A., (2003), Atmospheric microbiology in the northern Caribbean during African dust events, Aerobiologia, 19, p143-157.
[https://doi.org/10.1023/b:aero.0000006530.32845.8d]

-
Griffin, D.W., Kellogg, C.A., (2004), Dust storms and their impact on ocean and human health: dust in Earth’s atmosphere, EcoHealth, 1, p284-295.
[https://doi.org/10.1007/s10393-004-0120-8]

-
Li, C.-S., Huang, T.-Y., (2006), Fluorochrome in monitoring indoor bioaerosols, Aerosol Science and Technology, 40, p237-241.
[https://doi.org/10.1080/02786820500543308]

- Lighthart, B., Shaffer, B.T., (1997), Increased airborne bacterial survival as a function of particle content and size, Aerosol Science and Technology, 27, p439-446.
- Lighthart, B., Tong, Y., (1998), Measurements of total and culturable bacteria in the alfresco atmosphere using wetcyclone sampler, Aerobiologia, 14, p325-332.
-
Maki, T., Susuki, S., Kobayashi, F., Kakikawa, M., Yamada, M., Higashi, T., Chen, B., Shi, G., Hong, C., Tobo, Y., Hasegawa, H., Ueda, K., Iwasaka, Y., (2008), Phylogenetic diversity and vertical distribution of a halobacterial community in the atmosphere of an Asian dust (kosa) source region, Dunhuang city, Air Quality, Atmosphere and Health, 1, p81-89.
[https://doi.org/10.1007/s11869-008-0016-9]

-
Möhler, O., Demott, P.J., Vali, G., Levin, Z., (2007), Microbiology and atmospheric processes: the role of biologcal particles in cloud physics, Biogeosciences, 4, p1059-1071.
[https://doi.org/10.5194/bgd-4-2559-2007]

-
Jaenicke, R., (2005), Abundance of cellular material and proteins in the atmosphere, Science, 308, p73.
[https://doi.org/10.1126/science.1106335]

-
Jaenicke, R., Matthias-Maser, S., Gruber, S., (2007), Omnipresence of biological material in the atmosphere, Environmental Chemistry, 4, p217-220.
[https://doi.org/10.1071/en07021]

-
Jones, A.M., Harrison, R.M., (2004), The effects of meteorological factors on atmospheric bioaerosol concentrations - a review, Science of the Total Environment, 326, p151-180.
[https://doi.org/10.1016/j.scitotenv.2003.11.021]

-
Pratt, K.A., Demott, P.J., French, J.R., Wang, Z., Westphal, D.L., Heymsfield, A.J., Twohy, C.H., Prenni, A.J., Prather, K.A., (2009), In situ detection of biological particles in cloud ice-crystals, Nature Geoscience, 2, p398-401.
[https://doi.org/10.1038/ngeo521]

-
Prenni, A.J., Petters, M.D., Kreidenweis, S.M., Heald, C.L., Martin, S.T., Artaxo, P., Garland, R.M., Wollny, A.G., Pöschl, U., (2009), Relative roles of biogenic emissions and Saharan dust as ice nuclei in the Amazon basin, Nature Geoscience, 2, p402-405.
[https://doi.org/10.1038/ngeo517]

-
Sahu, A., Grimberg, S.J., Holsen, T.M., (2005), A static water surface sampler to measure bioaerosol deposition and characterize microbial community diversity, Journal of Aerosol Science, 36, p639-650.
[https://doi.org/10.1016/j.jaerosci.2004.10.001]

-
Sun, J., Ariya, P.A., (2006), Atmospheric organic and bioaerosols as cloud condensation nuclei (CCN): a review, Atmospheric Environment, 40, p795-820.
[https://doi.org/10.1016/j.atmosenv.2005.05.052]

-
Tong, Y., Lighthart, B., (2000), The annual bacterial particle concentrations and size distribution in the ambient atmosphere in a rural area of the Willamette valley, Oregon, Aerosol Science and Technology, 32, p393-403.
[https://doi.org/10.1080/027868200303533]

- Torrella, F., Morita, R.Y., (1981), Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater, Applied and Environmental Microbiology, 41, p518-527.
- Yamada, M., Iwasaka, Y., Kobayashi, H., Zhang, D., (2010), Challenge of measuring bioaerosols at kosa source areas: tethered balloon observation coupled with individual particle analysis, Earozoru Kenkyu, 25, p13-22.