Identification of Culturable Bioaerosols Collected over Dryland in Northwest China: Observation using a Tethered Balloon
Abstract
The transfer of microorganisms is important process for ecosystems. Microorganisms in dryland can transport itself to wetland through atmospheric diffusion, but only few papers reported about the atmospheric bioaerosol present over dryland. We carried out the direct sampling using a tethered balloon over Dunhuang City, China’s northwestern dryland. Bioaerosols were collected using a tethered balloon with a bioaerosol collector at 820 m above the ground (1,960 m above the sea level) around noon on August 17, 2007. The bioaerosols were cultured after the collection at Dunhuang Meteorological observatory. Two strains of molds were isolated using the Nutrient agar medium. About 400-bp 18S rRNA partial sequences were amplified by PCR and determined afterwards. The results of a homology search by 18S rRNA sequences of isolates in DNA databases (GenBank, DDBJ, and EMBL) and an observation of the form revealed that two bioaerosols in the convective mixed layer over Dunhuang City were Cladosporium sp. and Aspergillus sp.
Keywords:
Atmospheric bioaerosols, Cladosporium, Aspergillus, Dryland ecosystem, Tethered balloon1. INTRODUCTION
Drylands are extensive, covering 30% of the Earth’s land surface and 50% of Africa (Sankaran et al., 2005; Scholes and Archer, 1997). Furthermore, dryland ecosystems support a large fraction of the human population and most pastoralist societies (Trenton et al., 2010; Sankaran et al., 2005). Soil microbiology of dryland has been researched in the fields of biogeography and extremophiles (Friedmann et al., 1993; Friedmann, 1982; Friedmann and Kibler, 1980; Pielou, 1979). Friedmann and Kibler (1980) reported that the nitrogen source for endolithic microorganisms in deserts is abiotically fixed nitrogen, which is produced by atmospheric electric discharges (lightning or aurora) and conveyed to the rock by atmospheric precipitation. Based on their phylogenetic analysis of arid soils of the Loess Plateau, China, Kenzaka et al. (2010) reported that most phylotypes found in this dryland had low similarity with known strains in various phyla. The transfer of microorganisms from one place to another may lead to significant changes in ecosystems and the microorganisms in dryland will transport itself into wetland through atmospheric diffusion.
Interesting data were presented by Wang et al. (2010) who examined the seasonal variations of airborne bacteria found in the Mogao Grottoes, Dunhuang, China. However, their limitation lies in their location of the sampler which was mounted at 1.5 m above the ground level. Aiming to obtain wider range of atmospheric bioaerosol samples, we employed balloon-borne measurement (Chen et al., 2010a, b) to investigate the atmospheric mineral particles of Beijing. Using our tetheredballoon technique, we were able to collect atmospheric bioaerosols that can be found in the convective mixed layer over the dryland.
In this study, the atmospheric bioaerosols over the dryland in northwest China, Dunhuang, is investigated using a tethered balloon and bioaerosol sampler. The separated culture and identifications of the atmospheric bioaerosols were reported.
2. EXPERIMENTAL
2. 1 Location and Sampling Date
The sampling of the atmospheric bioaerosols using tethered balloon was made at Dunhuang (40°00′N, 94° 30′E, 1,140 m above sea level, Fig. 1), China, which is on the east side of the Tarim Basin (Taklamakan desert). Dunhuang is the location for atmospheric bioaerosol measurements over the dryland because the particulate matter originated from Taklamakan desert is frequently transported through combination of westerly wind and local circulations. The sampling was carried out around noon (13:20-14:20 Beijing Standard Time) on August 17, 2007. The weather of the sampling date was cloudy.
2. 2 Direct Sampling Method and Atmospheric Observations
The aerosol particle collection and the atmospheric observations were carried out using a tethered balloon which can lift instruments up to 1,000 m with maximum payload of 10 kg. As shown in Fig. 2, a bioaerosol sampler, an optical particle counter (OPC; KR-12A, Rion Co., Ltd.), and a thermo-hygrometer (EX-501, EMPEX Instruments, Inc.) were mounted on the tethered balloon. The altitudes of the balloon were monitored by the on-board global positioning system (GPS), and the data was transferred to the operating room on the ground by radio signal.
In this study, bioaerosols were collected on a 0.45μm pore-size membrane filter with the bioaerosol sampler. The filter was set into a filter holder (In-Line Filter Holder, 47 mm; Millipore Co., Ltd.) in the sampler under sterile condition and all of them were pre-autoclaved. The sampling was started at 820 m above the ground (1,960 m above the sea level) using a remote controller with a radio transmitter. The sampling was carried out for 60 min and the flow rate of an air pump in the sampler was 13.5 L min-1. To avoid contamination during non-sampling period, the inlet and outlet of the filter holder were closed by shutter valves of the sampler. The mechanical details of the sampler were described by Iwasaka et al. (2009).
In addition to the direct sampling of the bioaerosols, temperature and relative humidity were measured with a thermo-hygrometer. The OPC measured aerosol number concentrations with the diameters of the particles larger than 0.3, 0.5, 0.7, 1.0, 2.0 and 5.0 μm. These data were used to discuss the atmospheric structure from the ground to the sampling altitude.
2. 3 Separated Culture and Identification
The atmospheric bioaerosols were cultured after the collection in the clean booth to protect from contamination of the other microorganisms at Dunhuang Metrological Station (Kobayashi et al., 2007). The filter sample put on the plate contains the Nutrient agar medium (Difco BD Co. Ltd.). The microorganisms were observed using an optical microscope (E2T-C, Nikon Co., Ltd.). The DNA was extracted from the isolates on the plate using cell wall lytic enzyme, lysozyme, and proteinase K (Sigma-Aldrich). 18S rDNA for eukaryote was amplified by polymerase chain reaction (PCR) using primer F1 (5′-TGGTTGATCCTGCCAGAGG-3′) and R1 (5′-GGCTACCTTGTTACG ACTT-3′). PCR reaction mixture (vol. 20 μL) included the following: 4 μL of 5×Buffer, 1.6 μL of 10×dNTP (2.5 mM each, dATP, dCTP, GTP, dTTP), 0.2 μL of each primers (20 mM), 12.8 μL of sterile deionized H2O, 1 U of Prime-STAR DNA polymerase (TAKARA BIO INC. Co., Ltd.), 1 μL of DNA (~30 ng). The thermal cycler (Dice, TAKARA BIO INC. Co., Ltd.) was used under the following conditions for amplification: initial 2 min denatureation at 98°C; 35 cycle-10 s denaturation at 98°C, 10 s annealing at 54°C, and 1.5 min extension at 72°C; final 3 min extension at 72°C.
The DNA sequencing of cloned rDNA was determined by a genetic analyzer (Applied Biosystems Co., Ltd.), and the related species of the isolates were searched by BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/) to DNA databases (GenBank/EMBL/DDBJ). These sequence data of isolates have been submitted to the DDBJ database under accession numbers AB455104 and AB455105.
3. RESULTS AND DISCUSSION
3. 1 Atmospheric Observations
During the observation, the weather was first cloudy with stratocumulus which was formed in the boundary layer as shown in Fig. 2, and then the cloudiness was gradually decreased (Iwasaka et al., 2009). Due to the cloudy weather, the temperature and the relative humidity (R.H.) near the ground at the observation time were relatively lower (about 22°C) and higher (about 65%) than usual summer daytime, respectively. The observation was carried out in moderate wind conditions. Dust events were not reported in Dunhuang and even in the upwind areas before the observation.
The balloon took 17 minutes to reach the sampling height (820 m) with the ascent rate of about 36 m min-1, measuring temperature, R.H. and particle concentration simultaneously. Fig. 3 shows the vertical profiles of potential temperature, R.H. and mixing ratio during the balloon flight.
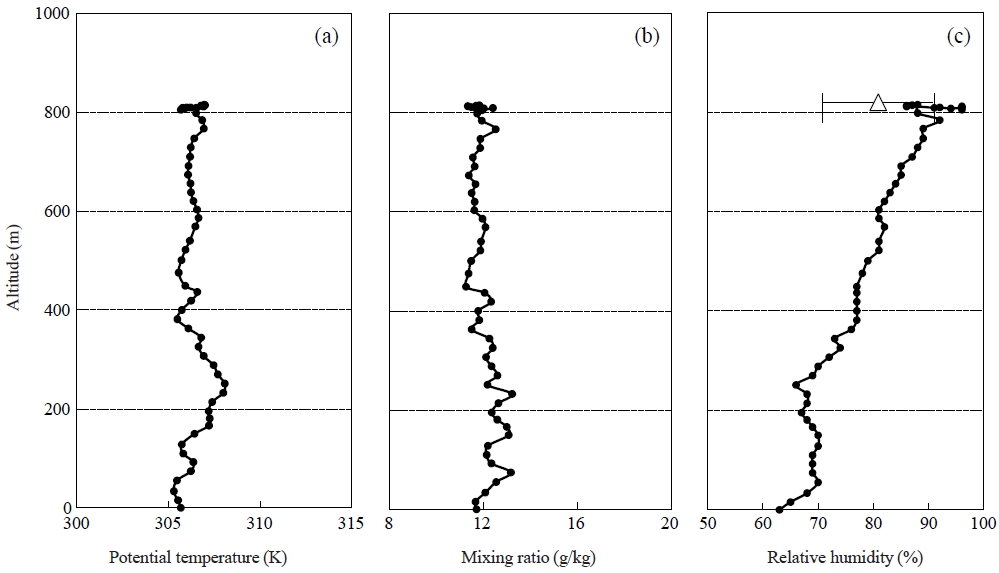
Vertical profiles of (a) potential temperature, (b) mixing ratio and (c) R.H. during the balloon flight. A symbol (△) and an error bar at the altitude of 820 m indicates average values and standard deviations during the aerosol collection, respectively.
The potential temperature increased at altitudes from 50 to 250 m, indicating that the atmosphere in this layer was stable. In contrast, the potential temperature decreased from 250 m to 400 m and kept almost constant above 450 m. From the profile of potential temperature, it is suggested that the vertical convection of the air is suppressed by the stable layer from 50 to 250 m. The air parcel above the stable layer would have a potential to be thermodynamically lifted to the sampling altitude. The values of the mixing ratio kept almost constant from the ground to the sampling altitude, but there were relatively large changes in the value below 450 m. It is likely that the air was well mixed, but below 450 m the air was influenced by small scale disturbance and it could cause the changes of mixing ratio.
The value of R.H. increased from 63 to 70% with the increase of altitude below 50 m, after which kept at 70% to 250 m altitude. The tendency of the vertical changes was the same as that of the potential temperature. The R.H. gradually increased from 250 m again, and reached nearly 100% at the sampling height. After that, the R.H. was decreased, and the aerosol sampling was conducted under the R.H. of 80%.
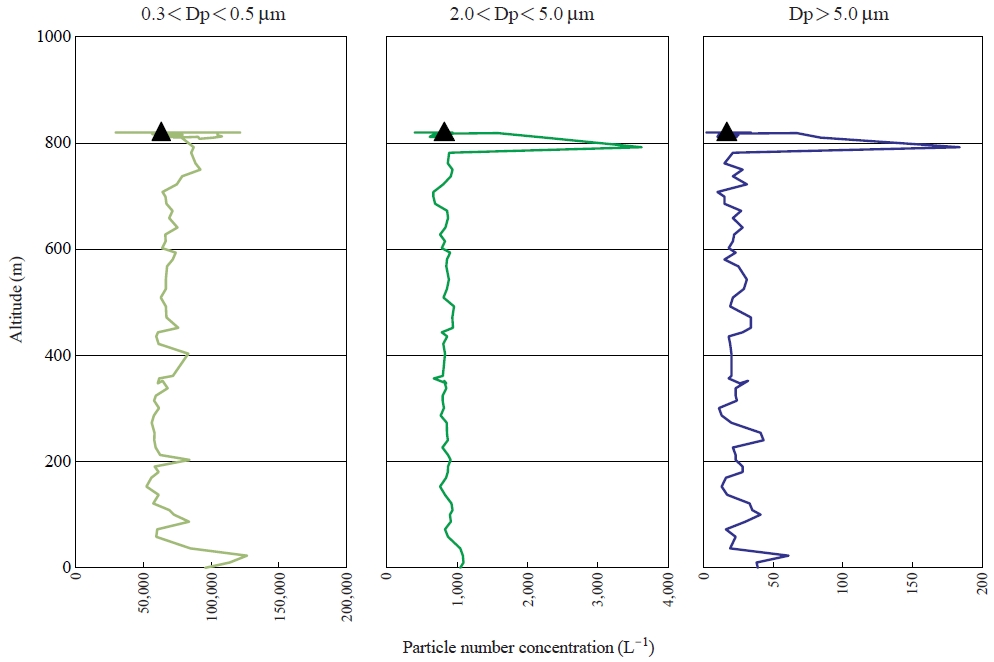
Fig. 4 shows the vertical profiles of the number concentrations of the particles in the size ranges of 0.3-0.5 μm, 2.0-5.0 μm and >5.0 μm. The particle concentrations were high below several tens meters, which correspond well with the altitude where the potential temperature and R.H. were changed clearly. It suggests that the aerosols emitted near the observation were trapped below the stable layer, which strongly reflects the influence of local aerosols. There were no clear differences of particle concentrations between 50 and 750m, although the different layers were appeared at the altitudes of 50-250 m and 250-450m(Fig. 3). At the altitude around 800 m, the concentration of the particles larger than 2.0 μm suddenly increased, but the smaller particles in the size range of 0.3-0.5 μm did not change considerably. Such concentration changes are frequently observed within clouds. Concerning the high R.H. around 800 m, the balloon should have encountered fragments or edge of the stratocumulus in the boundary layer as shown in Fig. 2. After passing the high concentration event of coarse particles, the concentrations immediately decreased to almost same value as that observed below 750 m, and the concentration did not change very much during the sampling. As a result, it can be mentioned that the atmosphere below 50 m represented the environment in the surface layer which was influenced by the local emission near the observation site.
3. 2 Separated Culture and Identification
We collected the bioaerosols directly at the altitude about 820 m above the ground (1,960 m above the sea level) under the atmospheric conditions as shown in Figs. 3 and 4. Two colonies grew overnight on the plate containing Nutrient agar medium. One colony was named BADHUN 0701 and the other one was named BADHUN 0702. Fig. 5 shows photographs of BADHUN 0701 (a) and BADHUN 0702 (b) on the plate after isolation. Two strains were observed to be of a kind of mold. BADHUN 0701 strain was black colony. In the case of BADHUN 0702 strain, the center of colony was brown and the circumference was white. These strains were observed by microscope. Fig. 6(a) and (b) show the microphotograph of mycelium (a) and conidia (b) of BADHU 0701 strain. The mycelium was branched, 2-4 μm wide. The conidia were shaped, about 6 to 7 and 3 to 4 μm in diameter. Fig. 6(c), (d), and (e) show the microphotograph of mycelium (c), conidiophores (d), and conidia (e) of BADHU 0702 strain. The mycelium was narrow and twisted, about 1 μm wide. The conidiophores were straight and small head-like swelling. The conidia were sphere, about 2 to 3 μm in diameter.

The colonies of atmospheric bioaerosols on the plates: BADHUN 0701 (a) and BADHUN 0702 (b) strains. Scale bar shows 1 cm.

Microphotograph of mycelium (a), conidia (b) of BADHU 0701 strain, mycelium (c), conidiophores (d), and conidia (e) of BADH 0702 strain. Scale bars show 20 μm in (a), (c), and (d); 10 μm in (b) and (e).
The 18S rDNA sequences of the fungus strains BADHU 0701 and 0702 were determined for identification under accession number AB455104 and AB455105, respectively. The similarities between its 18S rDNA sequence and DNA sequences in the Gen-Bank, DDBJ, and EMBL were researched using a homology search program package, BLAST (Altschul et al., 1997). The partial DNA sequence data of BADHU 0701 strain, 320 base pairs in length, was closely related to Cladosporium cladosporioides (DQ678004, 100.0%), Cladosporium cladosporioides (AF548071, 100.0%), and Cladosporium cladosprioides (AF548070, 100.0%), as shown in Fig. 7. Though BADHU 0701 strain was closely related to Cladosoprium cladosperioides from results of similarities, it belonged to the species of Cladosporium because it could not be identified as Cladosporium cladosporioides from the hyphae form (Fig. 6). BADHU 0701 was found Cladosporium sp. The partial DNA sequence data of BADHU 0702 strain, 396 base pairs in length, was closely related to Aspergillus versicolor (EU263603, 99.0%), Aspergillus sp. (DQ810193, 99.0%), Aspergillus versicolor (AF548069, 99.0%), Aspergillus versicolor (AF548068, 99.0%), Aspergillus silvaticus (AF548067, 99.0%), Aspergillus versicolor (AB008411, 99.0%), Aspergillus ustus (AB008410, 99.0%), Aspergillus ustus (AB002072, 99.0%), and Aspergillus sparsus (AB002066, 99.0%), as shown in Fig. 8. From the data of similarities, the colony form, micrographics, BADHU 0702 strain was belong to species of Aspergillus sp. (Figs. 5, 6, 8).

DNA sequence data of BADHU 0701 strain and similarities between its 18S rDNA sequence and DNA sequences in the GenBank, DDBJ, and EMBL.
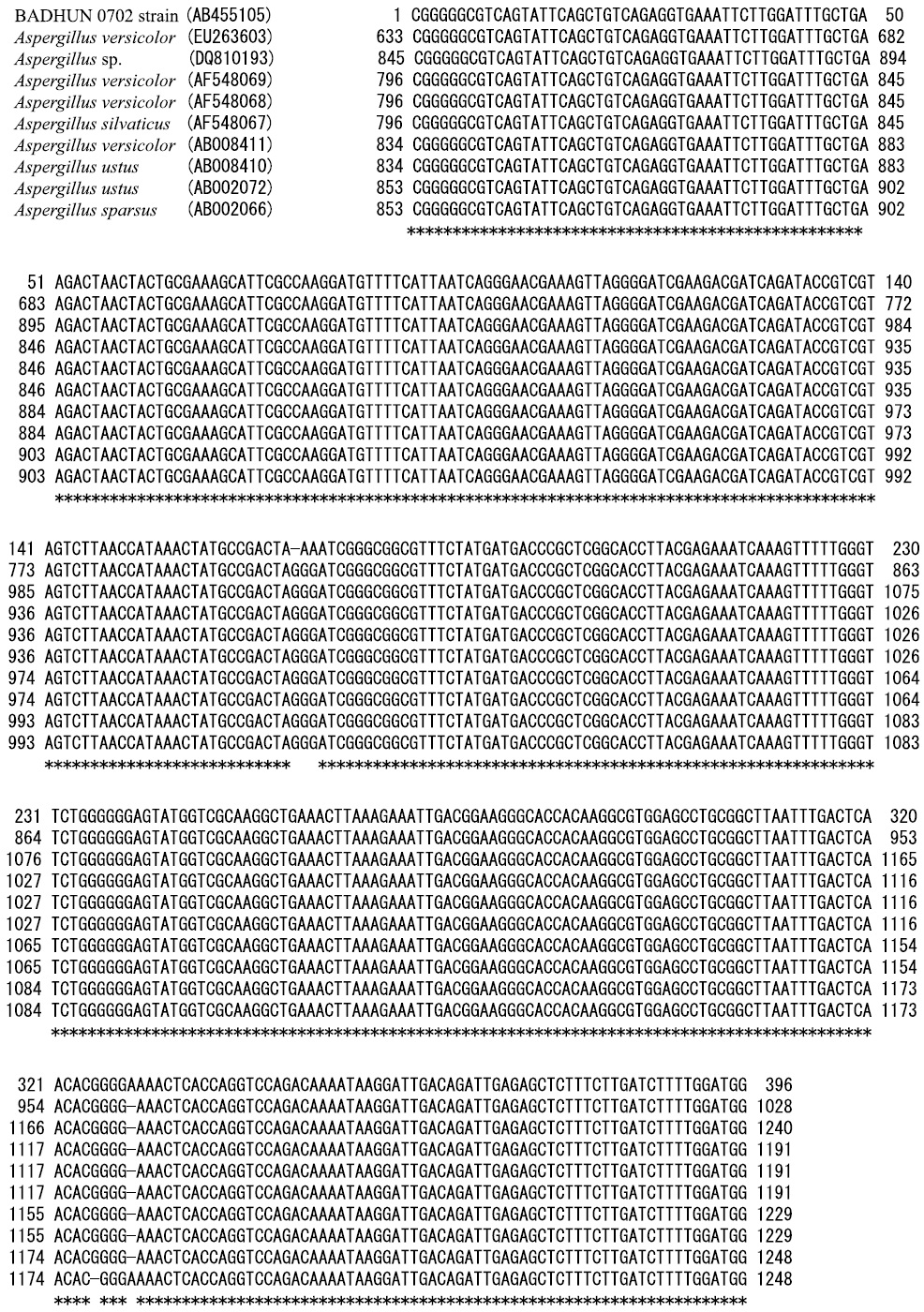
DNA sequence data of BADHU 0702 strain and similarities between its 18S rDNA sequence and DNA sequences in the GenBank, DDBJ, and EMBL.
It was found that there are Cladosporidium sp. and Aspelgillus sp. as living atmospheric bioaerosol at 820 m of altitude above the ground (1,960 m of altitude above the sea) over dryland, Dunhuang in northwest China. The authors reported in previous study that Bacillus subtilis and Bacillus atrophaeus were isolated from the sand near Dunhuang and that Bacillus cereus and Rhodosporidium sphaerocarpum were isolated from air sample at 50-100 m of altitude above the ground over Dunhuang (Kobayashi et al., 2007). Cladosporium and Aspergillus resulted in this study were reported as atmospheric bioaerosols (airborne microorganisms) over African desert by Griffin and Kellog (Griffin et al., 2007, 2006, 2003, 2001; Griffin and Kellogg, 2004; Kellogg et al., 2004). Microorganisms in this study agree with them over African dryland. Generally, the conidia of molds, i.e. Cladosporidium and Aspelgillus, have a tolerance for ultra-violet ray, dry, and low temperature. These characteristics seem to make these atmospheric bioaerosols able to live at high altitude.
4. CONCLUSIONS
To investigate atmospheric bioaerosols over the dryland, Dunhuang in northwest China, we examined direct sampling of them using a tethered balloon on August 17, 2007, the separated culture, and identifications. The atmospheric bioaerosol sample collected would reflect the air quality in the boundary layer and two strains were isolated. From the observation by microscope, DNA sequence of 18S rDNA, and the similarities search, two isolates were identified as Cladosporidium sp. and Aspergillus sp. The atmospheric observation pointed out the local scale distribution of bioaerosols through the convective mixing of the air, and suggested a possibility of regional scale diffusion of bioaerosols. In order to clarify dryland ecosystem, it will be necessary to research soil microbial community in Taklamakan desert in detail and the future study will be focused on it.
Acknowledgments
The authors are deeply grateful to the staff of Dunhuang Meteorological Station. In addition, we wish to thank Prof. Teruya Maki, Prof. Makiko Kakikawa, Prof. Takeshi Naganuma, Dr. Yutaka Tobo, and Dr. Chun-Sang Hong for their technical support in this study. A part of this research was supported by the Global Environment Research Funds (RF-072, B-0901, and C-1155) of the Ministry of the Environment, Japan, and the Grant-in-Aid for Scientific Research (A) (no. 20253005) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, of which another part was supported by the projects 2009DFA22650 and 2010DFA22770 of The Ministry of Science and Technology of China (MOST).
REFERENCES
-
Altschul, S.F., Madden, T.F., Schaffer, A.A., Zhang, J., Miller, W., Lipman, D.J., (1997), Gapped BLASTand PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Ressearch, 25, p3389-3402.
[https://doi.org/10.1093/nar/25.17.3389]

- Chen, B., Yamada, M., Shi, G., Zhang, D., Matsuki, A., Iwasaka, Y., (2010a), Vertical changes in mixing state of aerosol particles in the boundary layer in Beijing, China: Balloon-borne measurements in summer and spring, Journal of Ecotechnology, in press.
-
Chen, B., Shi, G., Yamada, M., Zhang, D., Hayashi, M., Iwasaka, Y., (2010b), Vertical change in extinction and atmospheric particle size in the boundary layers over Beijing: Balloon-borne measurement, Asian Journal of Atmospheric Environment, accepted.
[https://doi.org/10.5572/ajae.2010.4.3.141]

-
Friedmann, E.I., Kibler, A.P., (1980), Nitrogen economy of endolithic microbial communities in hot and cold deserts, Microbial Ecology, 6, p95-108.
[https://doi.org/10.1007/bf02010548]

-
Friedmann, E.I., (1982), Endolithic microorganisms in the Antarctic cold desert, Science, 215, p1045-1053.
[https://doi.org/10.1126/science.215.4536.1045]

-
Friedmann, E.I., Kappen, L., Meyer, M.A., Nienow, J.A., (1993), Long-term productivity in the cryptoendolithic microbial community of the Ross Desert, Antarctica, Microbial Ecology, 25, p51-69.
[https://doi.org/10.1007/bf00182129]

- Griffin, D.W., Garrison, V.H., Herman, J.R., Shinn, E.A., (2001), African desert dust in the Caribbean atmosphere: Microbiology and public health, Aerobiologia, 17, p203-213.
-
Griffin, D.W., Kellogg, C.A., Garrison, V.H., Lisle, J.T., Borden, T.C., Shinn, E.A., (2003), Atmospheric microbiology in the northern Caribbean during African dust events, Aerobiologia, 19, p143-157.
[https://doi.org/10.1023/b:aero.0000006530.32845.8d]

-
Griffin, D.W., Kellogg, C.A., (2004), Dust storm and their impact on ocean and human health: Dust in earth’s atmosphere, EcoHealth, 1, p284-295.
[https://doi.org/10.1007/s10393-004-0120-8]

-
Griffin, D.W., Westphal, D.L., Gray, M.A., (2006), Airbrne microorganisms in the African desert dust corridor over the mid-Atlantic ridge, ocean drilling program, Leg 209, Aerobiologia, 22, p211-226.
[https://doi.org/10.1007/s10453-006-9033-z]

-
Griffin, D.W., Kubilay, N., Kocak, M., Gray, M.A., Borden, T.C., Shinn, E.A., (2007), Airborne desert dust and aeromicrobiology over the Turkish Mediterranean coastline, Atmospheric Environment, 41, p4050-4062.
[https://doi.org/10.1016/j.atmosenv.2007.01.023]

-
Iwasaka, Y., Shi, G.-Y., Yamada, M., Kobayashi, F., Kakikawa, M., Maki, T., Nagatani, M., Chen, B., Tobo, Y., Hong, C.S., (2009), Mixture of Kosa (Asian dust) and bioaerosols detected in the atmosphere over the Kosa particles source regions with balloon-borne measurements, Possibility of long-range transport, Air Quality Atmosphere and Health, 2, p29-38.
[https://doi.org/10.1007/s11869-009-0031-5]

-
Kellogg, C.A., Griffin, D.W., Garrison, V.H., Peak, K.K., Royall, N., Smith, R.R., Shinn, E.A., (2004), Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa, Aerobiologia, 20, p99-110.
[https://doi.org/10.1023/b:aero.0000032947.88335.bb]

-
Kenzaka, T., Sueyoshi, A., Baba, T., Li, P., Tani, K., Yamaguchi, N., Nasu, M., (2010), Soil microbial community structure in an Asian dust source region (Loess Plateau), Microbes Environments, 25, p53-57.
[https://doi.org/10.1264/jsme2.me09164]

- Kobayashi, F., Kakikawa, M., Yamada, M., Chen, B., Shi, G.-Y., Iwasaka, Y., (2007), Study on atmospheric diffusion of bioaerosols in a KOSA source region, Earozoru Kenkyu, 22, p218-227.
- Pielou, E.C., (1979), Biogeography, ohon Wiley & Sons, New York, p42-46.
- Sankaran, M., Hannan, N.P., Scholes, R.J., Ratham, J., Augustine, D.J., Cade, B.S., Gignoux, J., Higgins, S.I., Roux, X.L., Ludwig, F., Argo, J., Banyikwa, F., Bronn, A., Bucini, G., Caylor, K.K., Coughenour, M.B., Diouf, A., Ekaya, W., Feral, C.J., February, E.C., Frost, P.G.H., Hiernaux, P., Hrabar, H., Metzger, K.L., Prins, H.H.T., Ringrose, S., Sea, W., Tew, J., Worden, J., Zambatis, N., (2005), Determinants of woody cover in African savannas, Nature, 438, p846-849.
-
Scholes, R.J., Archer, S.R., (1997), Tree-grass interactions in savannas, Annual Review of Ecology and Systematics, 28, p517-544.
[https://doi.org/10.1146/annurev.ecolsys.28.1.517]

- Trenton, E.F., Kelly, K.C., Jan, M.N., Ignacio, R.-I., Michael, A.C., (2010), An ecohydrological approach to predicting regional woody species distribution patterns in dryland ecosystems, Advances in Water Resources, 33, p215-230.
-
Wang, W., Ma, Y., Ma, X., Wu, F., Ma, X., An, L., Feng, H., (2010), Seasonal variations of airborne bacteria in the Mogao Grottoes, Dunhuang, China, International Biodeterioration and Biodegradation.
[https://doi.org/10.1016/j.ibiod.2010.03.004]