A Review of Photocatalytic Treatment for Various Air Pollutants
Abstract
Photocatalysis is a photochemical catalytic reaction which is a highly promising tool for the environmental cleanup process. It is very effective in treatment of environmental pollutants by its unique redox property. It has wide applications in the treatment of atmospheric pollutants (e.g., nitrogen dioxide, trichloroethylene, volatile organics, hydrogen sulfide, benzene, etc) through oxidative removal and by disinfection (aeromicro flora). In this research, the fundamental aspects of photocatalysis are described with respect to the composition of catalysts, experimental conditions (e.g., temperature, duration, etc), and interfering factors (e.g., catalyst deactivation).
Keywords:
Treatment of air, Pollution control, Photocatalysis, Oxidation of air pollutants1. INTRODUCTION
Photocatalysis is a rapidly expanding technology for the treatment of air pollutants. It can be defined as the mechanism leading to “acceleration of photoreaction with the aid of a catalyst”. The initial recognition on the heterogeneous photocatalysis was made when Fujishima and his colleagues discovered photolysis of water (Fujishima et al., 1972). In recent years, the use of semiconductor materials gained interests as photocatalytic medium for the removal of organic and inorganic species. This method has been suggested as a potent tool for environmental protection due to its great oxidation capacity (Robert and Malato, 2002). The photocatalysis is a very effective tool to process the environmental pollution. However, its application toward the treatment of the air pollutants is open widely. Hence, in this article, emphasis is given to describe its treatment efficiency in a number of aspects by surveying the uptodated knowledge in this field of research.
The catalyst can be used to induce oxidation and reduction of substrates simultaneously. UV light with higher energy band of <390 nm (or sunlight 390-700 nm) can be used as excitation energy. Heterogeneous photocatalysis using semiconductors (such as titanium dioxide (TiO2)) can be more effective than conventional waste treatment methods for removing organic species in the environment. The redox capacity of such media promotes the conversion of substrate to carbondioxide and water. This process is also called mineralization. Carbon dioxide is formed as the end product due to the oxidation of carbon atom of the reactant molecule. However, depending on the composition of reactant, the end product of the reaction can differ to a degree (Denny et al., 2007).
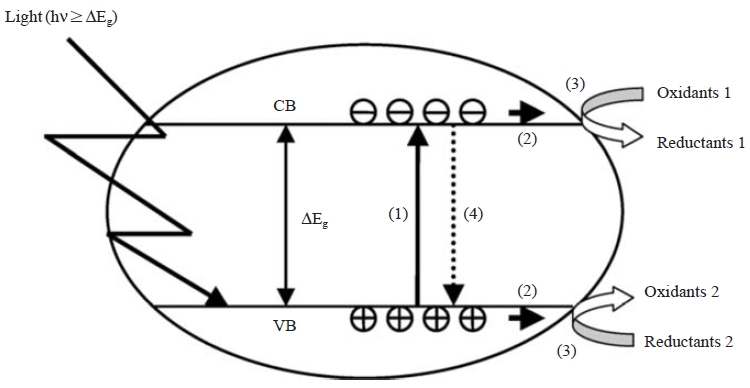
Photocatalysis with a semiconductor oxide (such as TiO2) is initiated by the absorption of a photon with energy equivalent to or greater than the band gap of the semiconductor (c.a. 3.2 eV for anatase). It then produces electrons and holes (e-/h+) in conduction band and valence band, respectively (Wilke and Breuer, 1999). Following the irradiation, the TiO2 particle can act either as an electron donor or acceptor for molecules in the surrounding medium. The electron and hole can also be recombined to release the absorbed light energy in the form of heat. When this recombination takes place, TiO2 cannot act as catalyst. Under such circumstances, both the redox process cannot take place. The valence band hole is strongly oxidizing due to the presence of OH radical, while the conduction band electron is strongly reducing due to the presence of O2- radical (Bahruji et al., 2010).
At the external surface, the excited electron on the conduction band and the hole on the valence band can take part in redox reactions. As the electron is excited from valence band to conduction band by absorption of energy, an empty positive hole is formed at valence band. When TiO2 comes in contact with water, the hydroxylation of hole takes place to form hydroxyl radical. The OH- radical adsorbed on valence band is anion form. As it has negative electrical charge, it is strongly attracted to positive hole. After being combined with hole, its negative charge is neutralized to form hydroxyl radical (•OH). This neutralized hydroxyl radical is very reactive in nature with strong affinity for electrons which facilitates the oxidation of other compounds. This hydroxyl radical (2.08) is the strongest oxidizing agent only after fluorine (3.06) (Datta et al., 2004).
The oxidation reactions are hence observed due to the presence of hydroxyl radical at valence band as well as other oxidizing agents, which are present in solution of reactants. An important reaction of the conduction band electron is the reduction of the adsorbed O2 to O2-. Thus, a redox environment is created by the presence of hydroxyl (oxidizing) and superoxide (reducing) radical over TiO2 surface. Upon the creation of the redox environment, the mineralization of desired component is observed, i.e., conversion of initial compound to final end product which is mainly carbondioxide, water, and organic acids (Liu et al., 2006) (Latasree et al., 2004).
1. 1 Characteristics of an Ideal Photocatalyst, Advantages of This Method
According to Fujishima et al. (2000), the ideal photocatalyst should possess the following properties.
- (a) Photo activity, low cost, and non-toxic nature: For large scale environmental clean up operations, the photocatalyst has to be available at low cost without toxic properties to flora and fauna when used at large concentrations.
- (b) Biological and chemical inertness: The catalyst used in the process of substrate treatment must not promote the formation of other complex and undesired substrates.
- (c) Stability towards photocorrosion: The catalyst must not undergo corrosion under prolonged light exposure; it has to be stable to tolerate the long light exposure which will be persistent throughout the process.
- (d) Suitability towards visible or near UV light: An excitation source which is essential for the initiation of reaction must be of low energy in the visible of near UV region so that the high radiation energy is not required.
1. 2 Various Steps in Photocatalysis Process
Heterogeneous photocatalysis is type of interaction where the reactant and catalyst exist in different phases. This type of reaction generally involves the following five steps (Shan et al., 2010): (i) diffusion of reactants to the surface of catalyst, (ii) adsorption of reactants onto the surface, (iii) reaction on the surface, (iv) desorption of products from the surface, and (v) diffusion of products from the surface (Devilliers, 2006) (Fig. 1).
2. PHOTOCATALYTIC MATERIALS
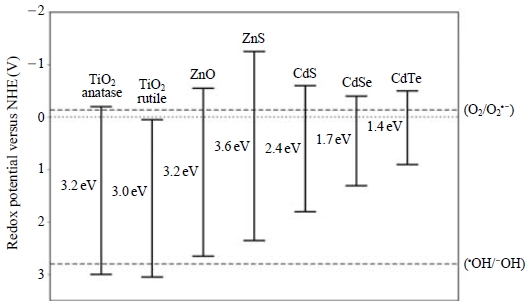
A wide range of semiconductors may be used for photocatalysis such as TiO2, ZnO, SnO2, WO3, Fe2O3, and CdS (Vinu and Madras, 2010). Among these semiconductor oxides, ZnO is generally unstable in illuminated conditions, especially at low pH values. WO3, although useful in the visible range, is less active photocatalytically than TiO2. Among others, the possibilities of CdS, ZnS, and iron oxides have been also tested. However, all of those materials have suffered from corrosive properties. Hence, TiO2 is yet the most useful material for photocatalytic purposes, owing to a number of advantages: exceptional optical and electronic properties, chemical stability, non-toxicity and low cost (Litter, 1999) (Fig. 2).
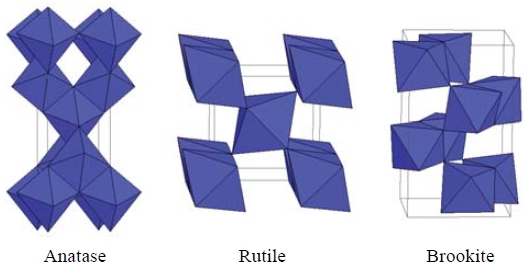
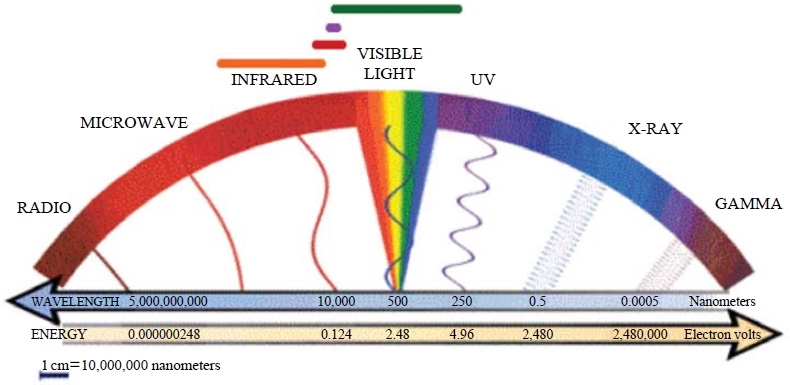
The commonest crystalline forms of TiO2 are anatase and rutile. The third form is brookite. Whose form is uncommon and unstable (It is stable only at high temperature). The structures of anatase, rutile, and brookite are presented in Fig. 3. However, the usage of TiO2 as a photocatalyst can also be limited by several factors. The most restrictive factor has been the need of UV (wavelength of <387 nm) as an excitation source due to its wide band gap (3.2 eV). Only 5 percent of visible radiation is available for this required excitation energy (Fig. 4). The photocatalytic method has wide applications in the waste water treatment, due to high availability of OH in water (Blanco et al., 2009). Although different in nature, its application to the treatment of various range of air pollutants is also reported. The catalyzing, deodorizing, adsorbing, and disinfection property of the TiO2 makes it a potential photocatalyst for the treatment of air pollutants.
Intensive studies are in progress to develop the existing materials or to prepare alternate materials for improved photocatalytic behaviour, which can be used under solar energy. To this end, the absorption of radiation must be shifted from UV to visible light region; it is active in UV region and is usually doped with a metal to structurally modify and increase the absorption capacity in the visible region. Visible light is renewable source with high potential, when employed at large scale treatment operations. The other demanding research area includes the modifications to increase the surface area of catalyst. The greater the surface area of the catalyst, the higher the extent of interaction takes place between the catalyst and reactant (Machado and charles, 2006). As a result, a greater efficiency in reaction can be acheived. Several studies reported that doping TiO2 with anions (such as carbon, nitrogen, sulfur, boron, and fluorine) can help shift the optical absorption edge of TiO2 towards lower energy (Li et al., 2005). Through such modification, one may increase the photocatalytic activity in visible light region. By the process of doping, the gap between valence and conduction band can be reduced with the formation of interbands between them (Juanru et al., 2007). Hence, less energy source (like visible radiation) can be sufficient for the excitation of electron from valence band to conduction band.
3. PHOTOCATALYSIS AND ITS APPLICATION TO THE TREATMENT OF AIR POLLUTANTS
Photocatalysis can be used as a very effective tool to treat air pollutants. It is also proved that this process is applicable in ambient environments, especially less humidified indoor environments (Jo and Kim, 2009). It has various advantages in comparison to the other conventional methods (Table 1). It has also been proven to deal with a wide range of air pollutants (Table 2). Here some pollutants and their treatment efficiency are taken as a reference to indicate its efficiency against different types pollutants released from various sources.
3. 1 Purification of Indoor Air and Gaseous Effluents
There are various indoor pollutants which can be responsible to cause various chronic health problems. Radons, formaldehyde, and VOC are common examples of indoor air pollutants. The number of aromatic VOC including benzene, toluene, and xylene (commonly called BTX) has been treated by the photocatalytic method (Pichat et al., 2000). The TiO2 photocatalytic treatment of toluene is known to yield benzaldehyde, with high applications in synthetic industry (Sleiman et al., 2009). TiO2 also has high capacity to deodorize the indoor air. The deodorization may be considered as the outcome of oxidation of the pollutant(Wei et al., 2010). A photo-reactor coated with TiO2 fiber glass mesh in the presence of artificial radiation light at approximately 365 nm (UV) is found to be very efficient in the mineralization of BTX compounds (Pichat et al., 2000). This oxidation can be further enhanced under the presence of strong oxidants (like ozone) in the indoor environmental conditions. Doping of TiO2 can further increase its catalytic efficiency (Park et al., 2004). VOCs in urban atmosphere are produced mainly by automobile exhausts. The majority of them are very stable recalcitrant compounds. TiO2-SiO2 based photocatalysis was thus employed for the removal of VOC (Zou et al., 2006). The catalyst can act as adsorbing as well as catalyzing agent. The amounts of hydrocarbons and carbondioxide are observed as the end products by such photocatalytic treatment of VOC. The presence of NO (along with VOC) showed some interfering effects (Ao et al., 2003). In addition, it is also reported that humidity as well reactive nature of NO with VOCs had huge influence on the progress of reaction.
3. 2 Removal of Trichloroethylene (TCE)
TCE is a very harmful widespread gaseous air pollutant, being carcinogenic in nature. When gas phase TCE is subjected to photodegradation in packed bed continuous flow reactor, it yielded many intermediate products like phosgene, dichloroacetyl chloride (DCAC), chloroform, hexachloroethane, alcohols, esters, aldehydes, carbon monoxide, and carbon dioxide (Wang et al., 2002). Phosgene emission can be reduced by using allopane clay suspended TiO2. Here, phosgene is adsorbed on to surface of allopane clay catalyst, and it can be degraded gradually (Nishikiori et al., 2011). TCE degradation mechanisms usually begin with the Cl- subtraction by the attack of hydroxyl radical of water present in air (Amama et al., 2001). TCE has also been removed by employing degussa titania coated reactor wall deposited on silica based support (Mohseni, 2005). A large amount of TCE can be removed by this process. The removal efficiency of TCE is very high due to the addition of silica support on degussa, as it provides higher interactive surface area for catalyst and reactant. The intermediates formed in the presence of silica-based support and in its absence can exhibit huge variations. Pure degussa titania interaction with TCE resulted in phosgene and dichloro acetyl chloride (DCAC) as end product (Mohseni, 2005). However, in the presence of silica based support, the DCAC was not formed. Instead, a very little amounts of carbon tetrachloride and chloroform were formed. As such, the efficiency of TCE photodegradation is very high, when TiO2 is doped with high valency cations (Park et al., 2004).
3. 3 Photocatalytic Reduction of NO2
NO2 is harmful and reactive compound and is emitted from various industrial sources (Latza et al., 2009). For removal of NO2, its photocatalytic reduction to NO by cuppric ion loaded thin TiO2 films was studied (Ohko et al., 2009). As cupric ion can induce photocatalytic reduction of NO2 to NO in air, it can also act as oxidizing agent to yield HNO3 from NO. As such, cuppric ion can be used to provide delicate redox conditions. However, oxidation efficiency of NO to HNO3 decreased, as the accumulation of HNO3 proceeds. In an alternative method, the photocatalytic oxidation of NO can take place under some specified hydrothermal conditions. The P25 degussa titania, commercially produced by a German company (Anatase and Rutile in ratio of 3 : 1), showed best oxidation of NO, if the catalyst is calcined at temperature of 200 degree for the duration of 24 hrs(Wu et al., 2008). The application of photocatalytic concrete material in urban places has also been very effective in the treatment of NO2 at highly polluted urban areas (Ballari et al., 2010).
3. 4 H2S Photo Degradation
Hydrogen sulphide is a colorless, poisonous, flammable gas with the characteristic foul odor of rotten egg (Kourtidis et al., 2008; Lambert et al., 2006). It often results from the bacterial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers (anaerobic digestion) (Chaiprapat et al., 2011). It also occurs in volcanic gases, natural gas, and some well waters. H2S can also be subject to photodegradation by chromium doped molecular seives in the presence of TiO2 (Portela et al., 2008). The chromium doped mesoporous crystalline molecular (Cr-MCM) sieves, prepared by hydrothermal method, are impregnated with TiO2. During the photodegradation, there was no oxidation of H2S to sulfurdioxide. However, the accumulation of sulphates deactivated the catalytic property of chromium. As another approach of H2S treatment, its photocatalytic decomposition was carried out in the presence of CdS catalyst. It allows the decomposition and photodegradation simultaneously with the production of H2 (Bai et al., 2010).
3. 5 Oxidation of Gaseous Benzene
Benzene is a flammable, colorless and carcinogenic compound (Bird et al., 2005). It is naturally produced, when wood is completely burned (during forest fires) or volcanic eruptions (Capaccioni et al., 2004). The commenest exposure to benzene is through the inhalation of cigarette smoke or car exhaust (Edwards and Jhantunen, 2001) (Weisel, 2010). This extremely toxic compound was discovered in the gas emitted by burning coal (The Lancet, 1904).
The photocatalytic oxidation of benzene can be carried out utilization of doped TiO2 catalysts (Zhong et al., 2007; Fu et al., 1995). The doped catalyst exhibited high decomposition of benzene than bare TiO2. It is highly reactive in the presence of sunlight, indicative of high potential in solar photocatalysis. The addition of Pd in to TiO2 increases photocatalytic activity by more than 2.3 times than pure TiO2 (Zhong et al., 2009).
3. 6 Photo Degradation and Detoxification of Chloranilines
Choloroaniline is a typical semi-volatile aromatic amine (Kataoka, 1996). It is precursor for various antibacterial products. It is strong irritant with the potential for tissue damage. The photocatalytic degradation of chloramines containing gases can be achieved very rapidly by TiO2 catalyst (An et al., 2011). Cholorobenzene and phenols are formed as intermediates of this degradation. Here the degradation as well as detoxification of the gaseous aromatic amines is simultaneously observed by this method.
3. 7 Microbial Disinfection in Air by Photocatalysis
Air usually contains high amount of micro-organisms. There are various airborne diseases which are transmitted through air (like tuberculosis, whooping cough, etc) (Martinez et al., 2008). The application of photocatalysis for the disinfection of microbes has been exercised. The Ag doped TiO2 acts as a very good disinfecting agent (Akhavan, 2009). The catalyst coated filters when passed over recirculation air, can induce disinfection of various microorganisms (e.g., Bacillus cereus, Staphylococcus aureus, Escherichia coli, and Aspergillus niger) which are used as indices for disinfecting property (Vohra et al., 2006). Photocatalysis is also very efficient in the inactivation of microbial growth. The principle of disinfection of photocatalyst can be expressed as the disintegration of cells by the activity of OH radical which oxidizes the intracellular components of micro-organisms (Cheng et al., 2007). Thus, this method can be very beneficial under rapidly infectious conditions.
4. CONCLUSIONS
This article is written to review basic principle and concept of photocatalysis and its application toward the treatment of air pollutants. A wide variety of air pollutants and photocatalytic methods that have been successful in their treatment was briefly described here. Although the application of photocatalysis was has been extended to cover many field conditions, the progress in this field of research is yet insufficient. However, based on the realization of its potential, a lot of photocatalytic applications and material modifications have been made for pollution control on a large scale. Yet a vast variety of atmospheric gases are not subjected to this method of treatment. In the near future, one needs to find a more favorable means to extend its applicability for the abatement of various air pollutants.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (No. 2010-0007876).
REFERENCES
-
Akhavan, O., (2009), Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation, Journal of Colloid and Interface Science, 336, p117-124.
[https://doi.org/10.1016/j.jcis.2009.03.018]

-
Amama, P.-B., Itoh, K., Murabayashi, M., (2001), Photocatalytic oxidation of trichloroethylene in humidified atmosphere, Journal of Molecular Catalysis A: Chemical, 176, p165-172.
[https://doi.org/10.1016/s1381-1169(01)00249-7]

-
An, T., Sun, L., Li, G., Gao, Y., Ying, G., (2011), Photocatalytic degradation and detoxification of o-chloroaniline in the gas phase: Mechanistic consideration and mutagenicity assessment of its decomposed gaseous intermediate mixture, Applied Catalysis B: Environmental, 102, p140-146.
[https://doi.org/10.1016/j.apcatb.2010.11.035]

-
Ao, C.-H., Lee, S.-C., Mak, C.-L., Chan, L.-Y., (2003), Photodegradation of volatile organic compounds (VOCs) and NO for indoor air purification using TiO2: promotion versus inhibition effect of NO, Applied Catalysis B: Environmental, 42, p119-129.
[https://doi.org/10.1016/s0926-3373(02)00219-9]

-
Bahruji, H., Bowker, M., Dickinson, A., Greaves, J., James, D., Millard, L., Pedrono, F., (2010), Sustainable H2 gas production by photocatalysis, Journal of Photochemistry and Photobiology A: Chemistry, 216, p115-118.
[https://doi.org/10.1016/j.jphotochem.2010.06.022]

- Bai, X.-F., Cao, Y., Wu, W., (2010), Photocatalytic decomposition of H2S to produce Hydrogen over CdS nanoparticles formed in HY-zeolite pore, Renewable Energy (Article in Press).
- Ballari, M.-M., Yu, Q.-L., Brouwers, H.-G., (2010), Experimental study of the NO and NO2 degradation by photocatalytically active concrete, Catalysis Today, 161, p165-180.
- Bird, M.-G., Greim, H., Snyder, R., Rice, J.-M., (2005), International symposium: Recent advances in benzene toxicity, Chemico-Biological Interactions, 153-154, p1-5.
-
Blanco, J., Malato, S., Alarcón, D., Gernjak, W., Maldonado, M.-I., (2009), Review of feasible solar energy applications to water processes, Renewable and Sustainable Energy Reviews, 13, p1437-1445.
[https://doi.org/10.1016/j.rser.2008.08.016]

-
Capaccioni, B., Taran, Y., Tassi, F., Vaselli, O., Mangani, G., (2004), Source conditions and degradation processes of light hydrocarbons in volcanic gases: an example from El Chichón volcano (Chiapas State, Mexico), Chemical Geology, 206, p81-96.
[https://doi.org/10.1016/j.chemgeo.2004.01.011]

-
Chaiprapat, S., Mardthing, R., Kantachote, D., Karnchanawong, S., (2011), Removal of hydrogen sulfide by complete aerobic oxidation in acidic biofiltration, Process Biochemistry, 46, p344-352.
[https://doi.org/10.1016/j.procbio.2010.09.007]

-
Cheng, Y.-W., Chan, R.-C.-Y., Wong, P.-K., (2007), Disinfection of Legionella pneumophila by photocatalytic oxidation, Water Research, 41, p842-852.
[https://doi.org/10.1016/j.watres.2006.11.033]

- Datta, C., Naidu, R., Yenkie, M.-K., (2004), Photo-oxidative degradation of synthetic organic pollutant p-nitrophenol, Journal of Scientific and Industrial Research, 63, p518-521.
- Demeestere, K., Dewulf, J., Langenhove, H.-V., (2007), Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air, Environmental Science and Technology, 37, p489-538.
-
Denny, F., Scott, J., Chiang, K., Teoh, W.-Y., Amal, R., (2007), Insight towards the role of platinum in the photocatalytic mineralization of organic compounds, Journal of Molecular Catalysis A: Chemical, 263, p93-102.
[https://doi.org/10.1016/j.molcata.2006.08.031]

- Devilliers, D., (2006), Semiconductor photocatalysis, Energia Centre for Applied Energy Research, 17, p1-4.
-
Edwards, R.-D., Jantunen, M.-J., (2001), Benzene exposure in Helsinki, Finland, Atmospheric Environment, 35, p1411-1420.
[https://doi.org/10.1016/s1352-2310(00)00359-9]

-
Fu, X., Zeltner, W.-A., Anderson, M.-A., (1995), The gasphase photocatalytic mineralization of benzene on porous titania-based catalysts, Applied Catalysis B: Environmental, 6, p209-224.
[https://doi.org/10.1016/0926-3373(95)00017-8]

- Fujishima, A., Honda, K., Kikuchi, S., (1969), Photosensitized electrolytic oxidation on TiO2 semiconductor electrode, J Chem Soc Japan (Kogyo Kagaku Zasshi), 72, p108-109.
-
Fujishima, A., Rao, N.-T., Tryk, A., (2000), TiO2 photocatalysts and diamond electrodes, Electrochimica Acta, 45, p4683-4690.
[https://doi.org/10.1016/s0013-4686(00)00620-4]

-
Jo, W.-K., Kim, J.-T., (2009), Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds, Journal of Hazardous Materials, 164, p360-366.
[https://doi.org/10.1016/j.jhazmat.2008.08.033]

- Juanru, H., Mingwei, L., Zhong, C., (2007), Advances in doping of titania photocatalytic catalysts, Industrial Catalysis, 15, p1-4.
- Kataoka, H., (1996), Derivatization reactions for the determination of amines by gas chromatography, Journal of Chromatography, 733, p19-34.
-
Kourtidis, K., Kelesis, A., Petrakakis, M., (2008), Hydrogen sulfide (H2S) in urban ambient air, Atmospheric Environment, 42, p7476-7482.
[https://doi.org/10.1016/j.atmosenv.2008.05.066]

- Lambert, T.-W., Goodwin, V.-M., Stefani, D., Strosher, L., (2006), Hydrogen sulfide (H2S) and sour gas effects on the eye, Science of the Total Environment, 367, p1-22.
-
Lathasree, S., Nageswara Rao, A., SivaSankar, B., Sadasivam, V., Rengaraj, K., (2004), Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions, Journal of Molecular Catalysis A: Chemical, 223, p101-105.
[https://doi.org/10.1016/j.molcata.2003.08.032]

-
Latza, U., Gerdes, S., Baur, X., (2009), Effects of nitrogen dioxide on human health: Systematic review of experimental and epidemiological studies conducted between 2002 and 2006, International Journal of Hygiene and Environmental Health, 212, p271-287.
[https://doi.org/10.1016/j.ijheh.2008.06.003]

-
Li, Y., Hwang, D.-S., Lee, N.-H., Kim, S.-J., (2005), Synthesis and characterization of carbon-doped titania as an artificial solar light sensitive photocatalyst, Chemical Physics Letters, 404, p25-29.
[https://doi.org/10.1016/j.cplett.2005.01.062]

-
Litter, M-I., (1999), Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems, Applied Catalysis B: Environmental, 23, p89-114.
[https://doi.org/10.1016/s0926-3373(99)00069-7]

-
Liu, J.-H., Yang, R., Li, S.-M., (2006), Preparation and application of efficient TiO2/ACFs photocatalyst, Journal of Environmental Sciences, 18, p979-982.
[https://doi.org/10.1016/s1001-0742(06)60025-9]

-
Machado, L.-C.-R., Charles, B., (2006), Floating photocatalysts based on TiO2 supported on high surface area exfoliated vermiculite for water decontamination, Catalysis Communications, 7, p538-541.
[https://doi.org/10.1016/j.catcom.2005.10.020]

-
Martinez, L., Blanc, L., Nunn, P., Raviglione, M., (2008), Tuberculosis and air travel: WHO guidance in the era of drug-resistant TB, Travel Medicine and Infectious Disease, 6, p177-181.
[https://doi.org/10.1016/j.tmaid.2007.10.004]

- Mohseni, M., (2005), Gas phase trichloroethylene (TCE) photooxidation and by product:photolysis vs. titania/silica based photocatalysis, Chemosphere, 59, p335-342.
-
Nishikiori, H., Furukawa, M., Fujii, T., (2011), Degradation of trichloroethylene using highly adsorptive allophane TiO2 nanocomposite, Applied Catalysis B: Environmental, 102, p470-474.
[https://doi.org/10.1016/j.apcatb.2010.12.028]

- Ohko, Y., Noguchi, H., Nakamura, Y., Negishi, N., Takeuch, K., (2009), Highly selective photocatalytic reduction of NO2 in air to NO using Cu2+ -loaded TiO2 thin films, Journal of Photochemistry and Photobiology A: Chemistry, 206, p27-31.
-
Park, S.-E., Joo, H., Kang, J.-W., (2004), Effect of impurities in TiO2 thin films on trichloroethylene conversion, Conversion of Solar Energy Materials and Solar Cells, 83, p39-53.
[https://doi.org/10.1016/j.solmat.2004.02.012]

-
Pichat, P., Disdier, J., Van, H.-C., Mas, D., Goutailler, G., Gaysse, C., (2000), Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis, Catalysis Today, 63, p363-369.
[https://doi.org/10.1016/s0920-5861(00)00480-6]

-
Portela, R., Canela, M.-C., Sánchez, B., Marques, F.-C., Stumbo, A.-M., Tessinari, R.-F., Coronado, J.M., Suárez, S., (2008), H2S photodegradation by TiO2/M-MCM-41 (M=Cr or Ce): Deactivation and by-product generation under UV-A and visible light, Applied Catalysis B: Environmental, 84, p643-650.
[https://doi.org/10.1016/j.apcatb.2008.05.020]

-
Robert, D., Malato, S., (2002), Solar photocatalysis: a clean process for water detoxification, The Science of the Total Environment, 291, p85-97.
[https://doi.org/10.1016/s0048-9697(01)01094-4]

-
Shan, A.-Y., Mohd, T.-I., Rashid, S.-A., (2010), Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review, Applied Catalysis A: General, 389, p1-8.
[https://doi.org/10.1016/j.apcata.2010.08.053]

-
Sleiman, M., Conchon, P., Ferronato, C., Chovelon, J.-M., (2009), Photocatalytic oxidation of toluene at indoor air levels (ppbv): Towards a better assessment of conversion, reaction intermediates and mineralization, Applied Catalysis B: Environmental, 86, p159-165.
[https://doi.org/10.1016/j.apcatb.2008.08.003]

- Smith, J., (2010), The TiO2 Group. University of Colorado, USA, (http://ruby.colorado.edu/~smyth/min/tio2.html).
- The Lancet, (1904), Benzene a poisonous content of coal gas, 163, p526-527.
- Vinu, R., Madras, G., (2010), Environmental remediation and photocatalysis, Journal of the Indian Institute of Science, 90, p189-229.
-
Vohra, A., Goswami, D.-Y., Deshpande, D.-A., Block, S.-S., (2006), Enhanced photocatalytic disinfection of indoor air, Applied Catalysis B: Environmental, 64, p57-65.
[https://doi.org/10.1016/j.apcatb.2005.10.025]

-
Wang, K.-H., Jehng, J.-M., Hsieh, Y.-H., Chang, C., (2002), The reaction pathway for the heterogeneous photocatalysis of trichloroethylene in gas phase, Journal of Hazardous Materials, 90, p63-75.
[https://doi.org/10.1016/s0304-3894(01)00331-4]

-
Wei, Z., Sun, J., Xie, Z., Liang, M., Chen, S., (2010), Removal of gaseous toluene by the combination of photocatalytic oxidation under complex light irradiation of UV and visible light and biological process, Journal of Hazardous Materials, 177, p814-821.
[https://doi.org/10.1016/j.jhazmat.2009.12.106]

-
Weisel, C.-P., (2010), Benzene exposure: An overview of monitoring methods and their findings, Chemico-Biological Interactions, 184, p58-66.
[https://doi.org/10.1016/j.cbi.2009.12.030]

-
Wilke, K., Breuer, H.-D., (1999), The influence of transition metal doping on the physical and photocatalytic properties of titania, Journal of Photochemistry and Photobiology A: Chemistry, 121, p49-53.
[https://doi.org/10.1016/s1010-6030(98)00452-3]

-
Wu, Z., Wang, H., Liu, Y., Gu, Z., (2008), Photocatalytic oxidation of nitric oxide with immobilized titanium dioxide films synthesized by hydrothermal method, Journal of Hazardous Materials, 151, p17-25.
[https://doi.org/10.1016/j.jhazmat.2007.05.050]

- www.spacetoday.org/DeepSpace/Telescopes/GreatObservatories/Chandra/ChandraSpectrum.htm, .
-
Zhong, J., Lu, Y., Jiang, W.-D., Meng, Q-M., He, X.-Z., Li, J.-Z., Chen, Y.-Q., (2009), Characterization and photocatalytic property of Pd/TiO2 with the oxidation of gaseous benzene, Journal of Hazardous Materials, 168, p1632-1635.
[https://doi.org/10.1016/j.jhazmat.2009.02.158]

-
Zhong, J., Wang, J., Tao, L., Gong, M., Zhimin, L., Chen, L., (2007), Photocatalytic degradation of gaseous benzene over TiO2/Sr2CeO4: Preparation and photocatalytic behavior of TiO2/Sr2CeO4, Journal of Hazardous Materials, 140, p200-204.
[https://doi.org/10.1016/j.jhazmat.2006.06.063]

-
Zou, L., Luo, Y., Hooper, M., Hu, E., (2006), Removal of VOCs by photocatalysis process using adsorption enhanced TiO2-SiO2 catalyst, Chemical Engineering and Processing, 45, p959-964.
[https://doi.org/10.1016/j.cep.2006.01.014]