A Case Study of Risk Assessment of Ozone Impact on Forest Tree Species in Japan
Abstract
Ozone (O3) is a main component of photochemical oxidants and a phytotoxic air pollutant. Although the current levels of tropospheric O3 in East Asia could adversely affect productivity of forest tree species, risk assessments of O3 impact were limited. In this paper, we summarize the methodology of risk assessment of O3 on forest tree species based on our two previous studies, risk assessments of O3 impact on the growth of Fagus crenata by Watanabe et al. (2012) and on the annual carbon absorption of three representative conifers, Cryptomeria japonica, Pinus densiflora and Larix kaempferi by Watanabe et al. (2010). O3 sensitivity of each tree species obtained from an experimental study, O3 exposure and atmospheric N deposition based on field monitoring and vegetation survey were integrated by geographic information system method. Based on the results, we conclude that the area with high risk of O3 impact does not necessarily correspond to the area with high O3 exposure. The varieties of tree habitat, tree sensitivity to O3 and annual carbon absorption among the tree species, and N deposition-induced change in the O3 sensitivity of F. crenata are raised as the factors of discordance between areas with high risk and those with high O3 exposure. In the last part of this paper, we discuss the present uncertainty and perspectives of risk assessment for the future studies on the impact of O3 on forest tree species in East Asia.
Keywords:
Sensitivity to ozone, Growth reduction, Geographic information system, Exposure to ozone, Nitrogen deposition1. INTRODUCTION
Tropospheric ozone (O3) is recognized as a widespread phytotoxic gaseous air pollutant, and the concentration has been increasing in the northern hemisphere (ADORC, 2006; Akimoto, 2003; Matyssek and Sandermann, 2003). In East Asia, relatively high concentrations of O3 have been frequently recorded not only in the suburbs of big cities, but also in several rural or mountainous areas (Network Center for EANET, 2009; Takeda and Aihara, 2007; Wang et al., 2006; Yoshikado, 2004). Furthermore, the emission of precursors for O3 in East Asian region has rapidly increased for several decades and this trend will continue in the near future (Yamaji et al., 2008; Ohara et al., 2007). Therefore, negative impact of O3 on forest tree species will be emphasized. To develop adequate measures for protecting functions of forest such as carbon sink, timber production and conservation of biodiversity, risk assessment of O3 impact on forest tree species is quite important. Especially, mapping of high risk areas is useful method for visual understanding and special analyses of the risk.
In Europe, the risk of O3 impact on forest trees had been discussed and assessed based on the concept of critical level over these two decades (Mills et al., 2010). The critical level of O3 for forest trees was calculated from the relationship between the growth of seedlings of O3-sensitive tree species and accumulated exposure or accumulated stomatal flux of O3 (Mills et al., 2010; Karlsson et al., 2007). The area exceeded critical level of O3 was mapped as high-risk areas throughout Europe (Simpson et al., 2007).
In Japan, concentrations of photochemical oxidants are officially monitored. Because O3 is the main component of photochemical oxidants, atmospheric concentration of O3 is also tabulated as that of photochemical oxidants under the Air Pollution Control Law Enforcement Regulations in Japan. In this paper, therefore, the concentration of photochemical oxidants is regarded as that of O3. Total number of monitoring stations in Japan is approximately 1200 (Fig. 1). The estimation of O3 concentration by model simulation was also developed recently (Yamaji et al., 2008; Tanimoto et al., 2005). Thus, the brief overview for the distribution of O3 concentration in Japan has been understood. On the other hand, the knowledge about the effects of O3 on forest tree species, especially in relation to ecophysiological traits, has been also obtained from the experimental studies although these experiments were mainly conducted with tree seedlings (Yamaguchi et al., 2011). Risk assessment of O3 on Japanese forest tree species was firstly reported by Takagi and Ohara (2003). They estimated the O3 impact on the growth of commercial conifer, Cryptomeria japonica, in Kanto region. Kohno et al. (2005) estimated the critical level of O3 for forest trees based on the experimental studies with 18 tree species, and mapped the area exceeded critical level of O3 corresponding to a 10% growth reduction of O3-sensitive tree species throughout Japan. Watanabe et al. (2010) reported risk assessment of O3 impact on the carbon absorption capacity by forests of Japanese representative afforestation conifers, C. japonica, Pinus densiflora and Larix kaempferi, in each prefecture of Japan. Watanabe et al. (2012) assessed the risk of O3 impact on the growth of Fagus crenata, a representative broad-leaved deciduous tree species in Japan, with consideration of change in the sensitivity to O3 associated with atmospheric nitrogen (N) deposition. On the other hand, risk assessment of O3 in East Asian countries other than Japan is quite limited, whereas high risk of O3 has been indicated in this region from global scale risk assessment (Sitch et al., 2007).
In this paper, to develop risk assessment of O3 impact on forest tree species in East Asian region including Japan, we summarize the methodology of risk assessment based on our two previous studies (Watanabe et al., 2012, 2010). In the following section, we define the O3 risk in this paper. The procedures for each component of the risk assessment are explained in sections 3-6. Section 7 shows some results of the risk assessment of Watanabe et al. (2012, 2010). In section 8, we discuss the present uncertainty and perspectives of risk assessment for the future studies.
2. DEFINITION OF OZONE RISK
Generally, risk assessment for chemical substances was comprised of evaluations of hazard characterization and exposure (WHO/IPCS, 2004). Although the hazard characterization includes many aspects of toxicity for the chemical substances, for simplify, it is defined the quantitative effect of one unit of O3 on target parameter. This term can be described as “O3 sensitivity” of the tree species. The whole-plant dry mass increment has been used as target parameter for the analysis of O3 sensitivity because this parameter is highly reflected by accumulating effects of O3 (Mills et al., 2010; Karlsson et al., 2007). To determine the sensitivity to O3, quantitative relationship between target parameter (i.e. the whole-plant dry mass increment) and O3 exposure should be established. The O3 exposure means the accumulated amount of O3 at an area of habitat for target plant. The AOTx (accumulated exposure over a threshold of x nmol mol-1) index has been developed in Europe, and is widely used as an O3 exposure index for evaluating the effects of O3 on tree species around the world (Yamaguchi et al., 2011; Kärenlampi and Skärby, 1996). The previous risk assessments of O3 for Japanese forest tree species also mainly used this index. The O3 risk is calculated as a product of sensitivity and exposure to O3, and thereby is expressed as relative value (e.g. % of growth reduction). This definition was applied in the risk assessment of O3 for F. crenata by Watanabe et al. (2012). On the other hand, Watanabe et al. (2010) estimated not only relative reduction in annual carbon absorption (ACA), but also absolute amount of O3-induced reduction in the ACA with a unit of Gg carbon year-1 as the risk of O3. It should be noted that the results of risk assessment do not indicate actual effect of O3 but potensial risk of O3 on forest tree production.
3. EVALUATION OF SENSITIVITY TO OZONE
Generally, exposure studies are applied for evaluating the sensitivity of tree species to O3. There are several different systems for O3 exposure experiment, including closed environmental control chamber, opentop chamber, free air O3 exposure system and so on. Multiple-year experiment is preferable because the effects of O3 on perennial plant such as trees would be carried over from the previous growing season to the next growing season (Yonekura et al., 2004).
The O3 sensitivities of the target tree species in Watanabe et al. (2012, 2010) were evaluated by experimental studies using open-top chambers. The details of the experiments were described in Watanabe et al. (2006) and Yamaguchi et al. (2007). Two or three-year-seedlings of F. crenata, C. japonica, P. densiflora and L. kaempferi were planted in 12 L pots filled with andisol. The seedlings were grown under 12 experimental treatment conditions, as determined by the combination of 4 gas treatments (charcoal-filtered air and 3 levels of O3 at 1.0, 1.5 and 2.0 times the ambient concentration) and 3 soil N treatments with NH4NO3 solution (0, 20 and 50 kg N ha-1 year-1) in open-top chambers during the two growing seasons. At the end of each growing season, dry mass of each plant organ of the seedlings was determined. The O3 concentrations in the chambers were monitored throughout the experimental period.
The exposure to O3 significantly reduced the whole-plant dry mass of all the tree species at the end of the experimental period (Yamaguchi et al., 2007; Watanabe et al., 2006). Interaction between O3 and N treatments for the whole-plant dry mass were found in F. crenata and L. kaempferi. The N supply enhanced O3 sensitivity of F. crenata, whereas the opposite response was found in L. kaempferi. There was no significant interaction between O3 and N treatments for the wholeplant dry mass of C. japonica and P. densiflora.
For F. crenata, the relationship between AOT40 and the whole-plant dry mass increment for a single growing season which was calculated as the difference in the whole-plant dry mass of the seedlings between the ends of the first and second growing seasons, was analyzed (Fig. 2a). The absolute value of slope for the regression line was considered as the sensitivity to O3. Watanabe et al. (2012) applied liner model for describing the O3 sensitivities of F. crenata as a function of N supply as shown in Fig. 2b. The equation for calculating relative growth reduction (RGred, %) as functions of AOT40 (μmol mol-1 h) and N deposition (TNdep, kg ha-1 year-1) for each habitat of F. crenata was as follows:
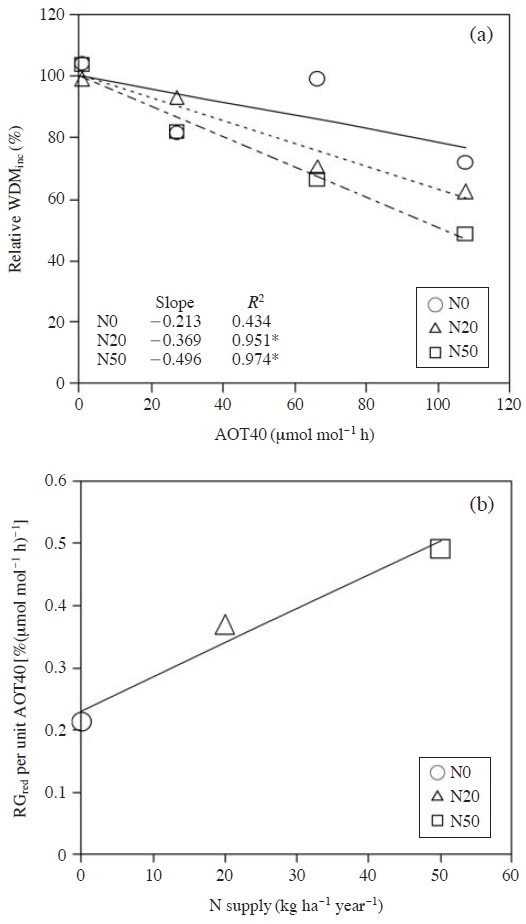
Relationships between AOT40 and relative whole-plant dry mass increment (WDMinc) (a) and between nitrogen supply and relative growth reduction per unit AOT40 (b) of Fagus crenata seedlings grown in the soil supplied nitrogen at 0 (N0), 20 (N20) and 50 kg ha-1 year-1 (N50). Regression line of (b): y=0.0055x+0.230; R2=0.967. This figure is adopted from Watanabe et al. (2012) with permission.
The necessity for consideration of N deposition-induced change in O3 sensitivity was also raised in the case of L. kaempferi. However, O3 sensitivities of L. kaempferi in the N treatment of 0 kg N ha-1 year-1 did not differ that in the N treatments of 20 kg N ha-1 year-1 (Watanabe et al., 2006). The 85% and 96% of L. kaempferi habitats in Japan have N depositions below 20 and 25 kg N ha-1 year-1, respectively, indicating the current levels of N deposition has little effect on the O3 sensitivity of L. kaempferi. Therefore, Watanabe et al. (2010) considered only single effect of O3 on L. kaempferi. In addition, they focused on the effect of O3 on forest carbon absorption, and the O3 exposure-response relationships for the ACA was analyzed. They determined carbon concentration in all organs and calculated the whole-plant carbon content of the seedlings. The ACA was calculated as the difference between the whole-plant carbon contents at the end of the first growing season and that of the second growing season. Fig. 3 shows O3 exposure-response relationships for the ACA of the three conifers. In this analysis, AOT60 was applied as an index for O3 exposure due to highest correlation with the ACA among AOTx (Watanabe et al., 2010). The absolute values of slope for the regression lines were calculated as relative reduction in the ACA per unit AOT60 (i.e. O3 sensitivity of ACA). The sensitivities of the ACA to O3 of L. kaempferi and P. densiflora were higher than that of C. japonica (Fig. 3).
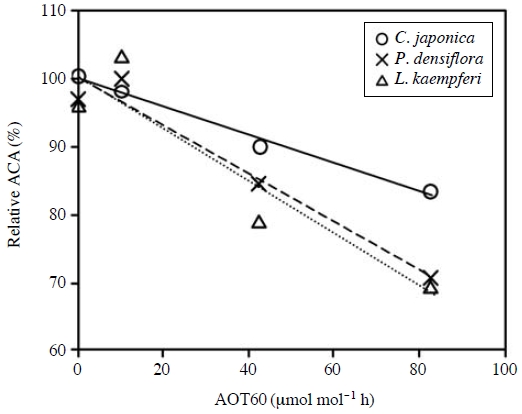
Relative annual carbon absorption (ACA) as a function of AOT60 for Cryptomeria japonica, Pinus densiflora and Larix kaempferi. Slope and R2 value of regression lines are -0.209 and 0.988 for C. japonica, -0.355 and 0.959 for P. densiflora, and -0.387 and 0.871 for L. kaempferi, respectively. Y intercepts of regression lines are adjusted as 100%. Data source: Watanabe et al. (2010).
The ACAs of C. japonica, P. densiflora and L. kaempferi in each prefecture in Japan were calculated with the following method. The dataset of forest resource assessment in Japan which is offered by Forestry Agency of Japan (2003) was used. This dataset contains the basal area (ha) and stand volume (m3) of each tree species in each forest age class from planting. The forest age class was delimited in 5 years, i.e. Class 1=1-5 years, Class 2=6-10 years etc. Stem volume per unit basal area (m3 ha-1) was calculated and converted to the carbon content of each organ (leaf, stem+branches, and root) per unit basal area (kg carbon ha-1) according to the method of Forestry and Forest Products Research Institute (2004). The carbon content of the whole-tree per unit basal area was calculated as the sum of carbon contents of leaf, stem+branch and root (Fig. 4a). The Gompertz curve (Hunt, 1982) was fitted to the relationship between forest age class and the whole- tree carbon content per unit basal area. Fiveyear carbon absorption per unit basal area (5-year CA, Gg carbon ha-1 5 year-1) as the difference between the whole-tree carbon content per unit basal area of an age class and that of the next age class was calculated based on the regression curve (Fig. 4b). The O3-induced reduction in the ACA per prefecture (Cred, Gg carbon year-1) of each forest age class was calculated by the following formula:

Example of the relationship between forest age class (Class 1=1-5 years, Class 2=6-10 years etc.) and carbon content of trees per area (Cryptomeria japonica in Tokyo Prefecture) (a), and model for calculation of O3-induced reduction in carbon absorption (b). Five-year carbon absorption per unit basal area (5-year CA) was calculated as the difference between the whole-tree carbon content per unit basal area (WCC) of an age class and that of the next age class based on the regression curve. Left figure is adopted from Watanabe et al. (2010) with permission.
where 1/5 is conversion coefficient from the 5-year CA to the ACA and R is the rate of reduction in the ACA per AOT60, which is the absolute value of the slope of the regression line between AOT60 and ACA (Fig. 3). This Cred indicates the reduction in the ACA at an AOT60 compared to that at zero AOT60. Finally, the Cred of all forest age classes were summed up.
4. ESTIMATION OF OZONE EXPOSURE
The concentrations of photochemical oxidants are officially monitored at approximately 1200 monitoring stations throughout Japan as mentioned above (Fig. 1). Watanabe et al. (2012, 2010) used this data set to evaluate O3 exposure throughout Japan. The number of hours in which the concentration of O3 is above either 0.06 μmol mol-1 (Num60) or 0.12 μmol mol-1 (Num120) is recorded by all the monitoring stations in Japan and available by the National Institute for Environmental Studies. However, hourly data concerning the O3 concentrations were available in approximately 40% of prefectures. Ishii et al. (2007) reported a high correlation (r=0.97) between the sum of Num60 and Num120 and the AOT40 over 12 h periods (0600-1800 hours) based on the monthly data between April and September, as calculated from available hourly O3 concentration data. Therefore, Watanabe et al. (2012) employed the method of Ishii et al. (2007) to estimate the AOT40 for all the monitoring stations in Japan. In the case of Watanabe et al. (2010), because they estimated the AOT60, modified equation of Ishii et al. (2007) was used. The maps of spatial distribution of AOT40 and AOT60 in Japan were created using the Geostatistical Analyst Extension of the ArcGIS 9.0 software (ESRI inc. USA). The kriging interpolation was applied for the estimation of AOT40 and AOT60 among the monitoring stations. Estimated distribution of AOT40 in Japan was shown in Fig. 5a. The highest AOT40 was distributed in the western part of the Kanto region. Relatively high AOT40 values were distributed not only in areas along the Pacific Ocean where there are many big cities, and also in the areas along the Sea of Japan, including the northern parts of the Chubu and Chugoku regions. The AOT60 showed similar trend with AOT40 (Watanabe et al., 2010).
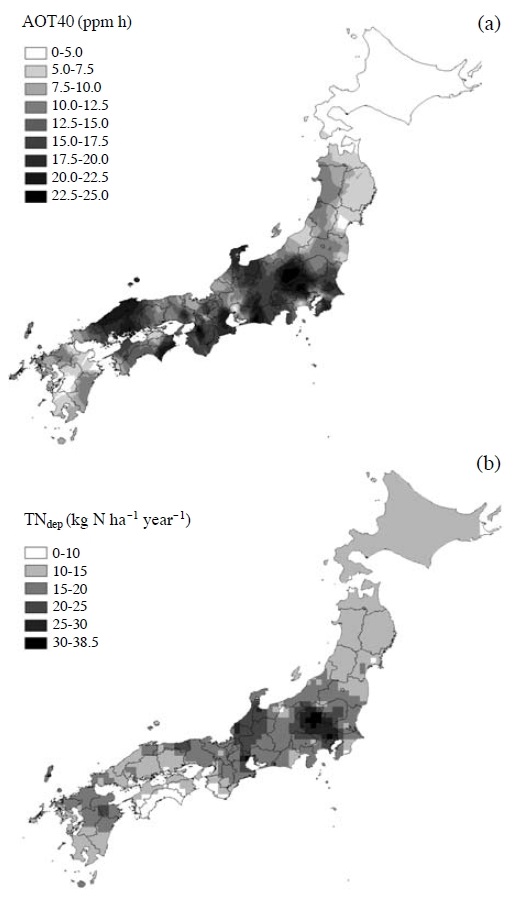
The distribution of the estimated AOT40 of O3 (a) and annual deposition of the total nitrogen (TNdep) (b) in Japan. The AOT40 was accumulated during 0600-1800 hours from April to September and averaged across 1999 to 2001. The TNdep was average across 1999 to 2001. This figure is adopted from Watanabe et al. (2012) with permission.
5. ESTIMATION OF ATMOSPHERIC NITROGEN DEPOSITION IN JAPAN
Watanabe et al. (2012) estimated atmospheric N deposition throughout Japan for the consideration of change in the O3 sensitivity of F. crenata with different atmospheric N deposition. In general, atmospheric N deposition is classified into wet and dry depositions. The data on wet depositions of NO3- and NH4+ were obtained from the Ministry of the Environment and Environmental Laboratories Association of Japan, which had approximately 100 monitoring stations in total (Ministry of the Environment, 2004; Environmental Laboratories Association, 2003). The distribution of wet deposition of N in Japan was estimated from these data set. Watanabe et al. (2012) applied the method for estimating dry deposition of N according to Fujita (2004). This method can estimate atmospheric concentration of N compounds from wet deposition, amount of precipitation and washout ratio (the ratio of concentration of a compound in precipitation to that in atmosphere), and thereby flux of dry deposition. This method is relatively easy to apply to estimate dry deposition of N in large area (e.g. nationwide scale). The washout ratio was obtained from the dataset of Environmental Laboratories Association (2003). A constant deposition velocity according to Puxbaum and Gregori (1998) was used in the estimation. The dataset for estimating dry deposition of N was the same with that for estimating wet deposition. To create the map of atmospheric N deposition, the inverse distance weighted (IDW) method was applied for estimating values of wet and dry deposition of N among the monitoring stations. Total N deposition (TNdep) was calculated as the sum of wet and dry depositions of N in the GIS software. As shown in Fig. 5b, relatively high TNdep was estimated in the western parts of the Kanto and Chubu regions. The average TNdep for Japan was 14.8 kg ha-1 year-1 and average ratio of dry deposition to wet deposition was 0.88.
6. HABITATS OF EACH TREE SPECIES IN JAPAN
The habitats of F. crenata, C. japonica, P. densiflora and L. kaempferi in Japan were determined from rasterized vegetation data (45′′×30′′ per mesh) of the National Survey on the Natural Environment, conducted by the Ministry of the Environment. These data were obtained from the Japan Integrated Biodiversity Information System (http://www.biodic.go.jp/J-IBIS.html). Geographical meshes containing the vegetation code for each species were taken to be their habitats. For F. crenata, the AOT40 and TNdep in each F. crenata habitat were extracted from the above-mentioned AOT40 and TNdep map, and were used to the calculation of RGred. For C. japonica, P. densiflora and L. kaempferi, the AOT60 in their habitats including plantation areas in each prefecture were averaged and used for the calculation of Cred.
7. DOES THE AREA WITH A HIGH RISK OF OZONE IMPACT CORRESPOND TO THE AREA WITH HIGH OZONE EXPOSURE?
Fig. 6a illustrates estimated O3-induced RGred with consideration of N deposition for F. crenata in Japan. Relatively high RGred values were estimated across a relatively wide area comprising the northern part of the Chubu region and the northwestern part of Kanto region. The estimated RGred values for the western part of the Kanto region, the southern parts of the Chubu and Kinki regions, and the central part of the Chugoku region were also higher than those for the other areas. The average and maximum estimated RGred values for Japan were 3.2% and 9.7%, respectively. Overall, the RGred for F. crenata was higher at the area with higher AOT40. However, it had 20-30% variation in the same AOT40 values (Watanabe et al., 2012). As shown in Fig. 6b, when the TNdep was assumed to be zero, the average and maximum estimated RGred values for Japan were 2.3% and 5.7%, respectively. Thus, the average and maximum estimated RGred values increased by 38% and 71%, respectively, when atmospheric N deposition was considered.
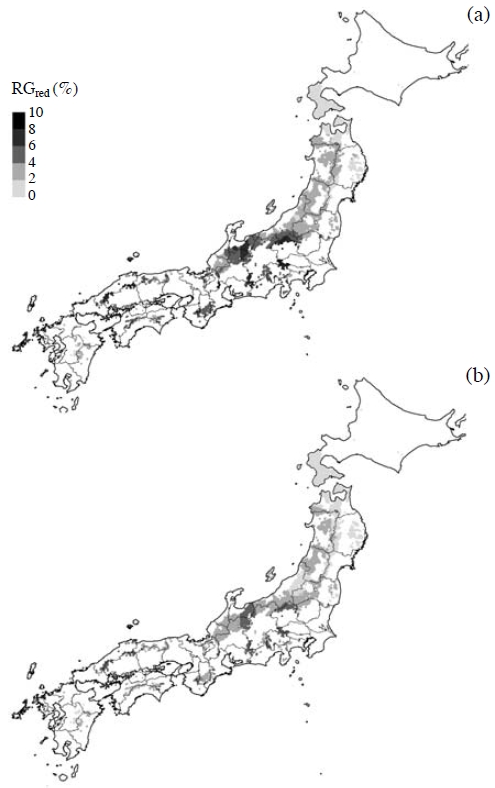
The distributions of O3-induced relative growth reduction (RGred) of Fagus crenata in Japan with consideration of nitrogen deposition (a) and without consideration of nitrogen deposition (b), which was estimated based on the RGred per unit AOT40 at 0 kg ha-1 year-1 of annual deposition of the total nitrogen. This figure is adopted from Watanabe et al. (2012) with permission.
The sum of estimated O3-induced reduction in the ACA(Cred) of C. japonica, P. densiflora and L. kaempferi was illustrated in Fig. 7. The Cred in Nagano, Gunma, Akita and Iwate Prefectures were relatively high as compared with those in the other prefectures. The areas with relatively high AOT60 of O3 did not necessarily correspond to areas with high Cred. Although the data of O3 exposure in Nagano, Akita and Iwate Prefectures were not as high as those in the other prefectures, the estimated Cred in these prefectures were relatively high (Figs. 5 and 7). In Nagano and Iwate Prefectures, it was mainly attributed to the widespread distribution of O3 sensitive species L. kaempferi and P. densiflora (Fig. 3). In Akita Prefecture, on the other hand, C. japonica was the primary tree species. The rate of the Cred of C. japonica in this prefecture was relatively low because of the relatively low O3 exposure and high O3 tolerance of C. japonica (Figs. 3 and 5). However, the ACA of C. japonica in Akita Prefecture was highest among all the prefectures. Therefore, we consider that even relative reduction in the ACA in Akita Prefecture is small, total amount of O3-induced reduction in the ACA (i.e. Cred) became high because of high ACA. Similar effect also contributed to the high Cred of P. densiflora in Iwate Prefecture and that of L. kaempferi in Nagano Prefecture.
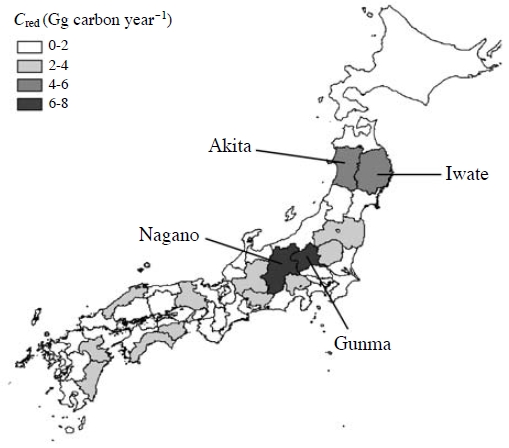
The estimated O3-induced reduction in the annual carbon absorption (Cred) in Japan. The values were the sum of the Cred of Cryptomeria japonica, Pinus densiflora and Larix kaempferi. This figure is adopted from Watanabe et al. (2010) with permission.
Based on the results from two risk assessments, we conclude that the area with a high risk of O3 impact does not necessarily correspond to the area with high O3 exposure. The varieties of tree habitat, tree sensitivity to O3 and ACA among the tree species, and atmospheric N deposition-induced change in the O3 sensitivity of F. crenata were raised as the factors of discordance between areas with high risk and those with high O3 exposure. To assess the risk of O3 impact on Japanese forest tree species, therefore, we must take into account not only the O3-exposure but also the differences in the response to O3, habitat and the ACA among the tree species.
8. UNCERTAINTY OF RISK ASSESSMENT AND PERSPECTIVES FOR FUTURE STUDY
The risk assessments that introduced in this paper integrated broad information such as O3 sensitivity of each tree species obtained from an experimental study, O3 exposure and atmospheric N deposition based on field monitoring and vegetation survey by GIS method. Similar or broader study has been systematically developed in Europe under Convention on Long-range Transboundary Air Pollution. We should develop our risk assessment for O3 impact on forest tree species in East Asia because tree species, climate, soil traits in East Asia are quite different from those in Europe.
Recently, the risk of O3 has been assessed based on the index of accumulated stomatal flux of O3, while the exposure index such as AOTx was retained as the recommended method for calculating critical levels for European forests (Mills et al., 2010; Matyssek et al., 2007; Simpson et al., 2007; Emberson et al., 2000). In fact, several researchers in European countries try to explain the difference of O3 sensitivities with different environmental conditions by the difference of stomatal O3 flux (Karlsson et al., 2007). At the present time, the information on the stomatal uptake of O3 for forest trees in Japan is very limited (Hoshika et al., 2009). Furthermore, although a part of O3 absorbed through the stomata is detoxified by antioxidative systems in the leaves or needles, quantitative evaluation of capacity for detoxification of O3 is future work worldwide (Fuhrer and Booker, 2003; Massman et al., 2000). The O3 sensitivities of F. crenata and L. kaempferi were changed by N load (Yamaguchi et al., 2007; Watanabe et al., 2006). This phenomenon is a result of many processes such as absorption and detoxification of O3, and acclimation including the repair of O3 damage. These processes may be affected not only by N load, but also by the other environmental stresses such as drought and elevated CO2 (Matyssek and Sandermann, 2003). Therefore, understanding and modeling of the effects of environmental changes on each process and their difference among the tree species are needed for accurate risk assessment of O3 for forest tree species in East Asia.
The sensitivity of tree growth to O3 was evaluated by the experiment with seedlings in the open-top chamber in the experimental studies of Watanabe et al. (2012, 2010). There may be differences in the sensitivities to O3 between seedlings and mature trees, and in environmental conditions between open-top chamber and field (Matyssek et al., 2007; Karnosky et al., 2003). The difference of O3 sensitivities between juvenile seedlings and mature trees is not clarified, while growth sensitivity to O3 of mature Fagus sylvatica trees evaluated with free air O3 exposure experiment (Pretzsch et al., 2009) was comparable to that of juvenile seedlings evaluated with open-top chamber experiments (Karlsson et al., 2007). Analysis of the O3 effects by multiple regression analysis with the data of long-term monitoring for growth of trees and environmental factors including O3 concentration would be another useful method for risk evaluation of mature trees (Karlsson et al., 2006).
We should consider about the variations of O3 sensitivity not only for inter-species, but also for intra-species. For example, variations such as individual leaf area and physiological traits have been reported among F. crenata genotypes in Japan (Koike and Maruyama, 1998; Hagiwara, 1977). These genetic variations of F. crenata may have an uncertainty for risk assessment of O3 because Paludan-Müller et al. (1999) reported that the sensitivities to O3 of F. sylvatica seedlings differed among the provenances in Europe. We did not have any information on the genetic variability in the sensitivities to O3 among the genotypes for Japanese forest tree species. In the near future, therefore, the comparison of O3 sensitivity among the genotypes is needed to improve the quality of the risk assessment of O3 impact.
As mentioned in the section of Introduction, the brief overview for the distribution of O3 concentration in Japan has been demonstrated. However, there are several points that should be improved. Hourly data concerning the O3 concentrations were only available in approximately 40% of prefectures. The monitoring stations for O3 in Japan have been mainly located in the urban areas because the aim of monitoring is the protection of human health. There is a limited number of monitoring stations in the mountainous and rural areas of Japan. However, O3 concentration in mountainous and rural areas were sometimes higher than that in urban region (Yamaguchi et al., 2010). Furthermore, diurnal variation of atmospheric O3 concentrations in the mountainous areas is generally different from that in urban areas (mainly flatland). Especially, little change in the O3 concentration under inversion layer is typical phenomenon in the mountainous areas. This phenomenon makes a concern when we estimate AOTx based on the Num60 and Num120 according to Ishii et al. (2007). For example, two constant O3 concentrations at 70 and 100 nmol mol-1 show the same Num60 and Num120, but different AOT40. Reconsidering of the location of monitoring station for O3 and availability of hourly data are needed for accurate assessment of O3 impact on forest trees in Japan. By this reconsidering, we also expect the development of the above-mentioned study on the estimation of O3 effects on growth of mature trees under field conditions by analyzing relationship between growth and O3 concentration (Karlsson et al., 2006).
Risk assessments introduced in this paper were based on the many monitoring data on O3 and N deposition and the data on vegetation surveys throughout Japan. For several countries in East Asian region, the number of monitoring site and vegetation surveys are limited. Atmospheric model simulation might be better as primary method estimating O3 concentration in rural sites (Yamaji et al., 2008; Tanimoto et al., 2005). Remote sensing from satellite have a possibility to be a tool to clarify the distribution of tree species (Katoh, 2004). Monitoring sites especially related to Acid Deposition Monitoring Network in East Asia (EANET) will play an important role in validation for estimated O3 concentration by model simulation and evaluation of vegetation by satellite remote sensing.
At the present time, routine method for measuring dry deposition of gases and particles has not yet established, while the method of monitoring for wet deposition is almost completly establishied. Watanabe et al. (2012) applied simple method for estimating atmospheric dry deposition of several N compounds using the data obtained from nationwide researches and constant value of Vd (Fujita, 2004; Ministry of the Environment, 2004; Environmental Laboratories Association, 2003; Puxbaum and Gregori, 1998). However, the Vd changes with changing environmental factors such as wind speed and air temperature. The development of ideal method for estimating dry deposition of N that can apply to nationwide scale is needed in the near future (Matsuda, 2008).
9. CONCLUSIONS
Current levels of O3 in East Asia could be enough to reduce the production of O3-sensitive forest tree species grown in the areas with relatively high O3 concentration during the growing season. Furthermore, because the gradual increase of O3 concentration in the near future is predicted, negative effects of O3 on forest tree species will be a great threat. Therefore, it is an urgent necessity to clarify the tree response to O3 and the other related environmental factor in forested areas for adequate risk assessment of O3 impact on forest tree species in East Asia. We expect that this paper will help to develop a framework for the future risk assessment of O3 impact on trees grown in East Asia.
Acknowledgments
This study was partly supported by the Ministry of the Environment, Japan through the Global Environmental Research Fund (C-03-07) and Environment Research and Technology Development Fund (B-1105), and by the Japan Society for the Promotion of Science through the Grant-in-Aid for Scientific Research B (23380078). We thank to the permission from Springer Science+Business Media for the reprint of figures.
REFERENCES
- ADORC (Acid Deposition and Oxidant Research Center), (2006), Tropospheric ozone: A growing threat, ADORC (Acid Deposition and Oxidant Research Center), Niigata, Japan, p26.
-
Akimoto, H., (2003), Global air quality and pollution, Science, 302, p1716-1719.
[https://doi.org/10.1126/science.1092666]

-
Emberson, L.D., Ashmore, M.R., Cambridge, H.M., Simpson, D., Tuovinen, J.-P., (2000), Modelling stomatal ozone flux across Europe, Environmental Pollution, 109, p403-413.
[https://doi.org/10.1016/s0269-7491(00)00043-9]

- Environmental Laboratories Association, (2003), The third report of the acid rain monitoring in Japan, Journal of Environmental Laboratories Association, 28, p126-185, (In Japanese).
- Forest Agency of Japan, (2003), Forest resource assessment, Forest Agency of Japan, http://www.rinya.maff.go.jp/j/keikaku/genkyou/index.html Accessed 25 June 2011, (In Japanese).
- Forestry and Forest Products Research Institute, (2004), Upgrading of evaluation for the budget of Carbon dioxide in forests and oceans, Forestry and Forest Products Research Institute, Tsukuba, p155, (In Japanese).
-
Fuhrer, J., Booker, F., (2003), Ecological issues related to ozone: agricultural issues, Environment International, 29, p141-154.
[https://doi.org/10.1016/s0160-4120(02)00157-5]

- Fujita, S., (2004), Study on the deposition of gaseous and particulate substances in East Asia, Journal of Japan Society for Atmospheric Environment, 39, p107-118, (In Japanese with English summary).
- Hagiwara, S., (1977), Cline of leaf area in Fagus crenata, Plant Species Biology, 1, p39-51, (In Japanese).
- Hoshika, Y., Hajima, T., Shimizu, Yo., Takigawa, M., Omasa, K., (2009), Effect of growing season on ozone stomatal flux for deciduous forests in East Asia, Eco-Engineering, 21, p3-8, (In Japanese).
- Hunt, R., (1982), Plant growth curves, Edward Arnold, London, p248.
- Ishii, T., Matsumura, H., Hayami, H., Kohno, Y., (2007), Relationship between oxidant-based AOT40 and forest environmental conditions of damaged Japanese cedars in the Kanto plains, Journal of Global Environment Engineering, 12, p51-62.
- Kärenlampi, L., Skärby, L., (1996), Critical levels for ozone in Europe: testing and finalizing the concepts, UN-ECE workshop reports, Department of Ecology and Environmental Science, University of Kuopio, Finland, p363.
-
Karlsson, P.E., Braun, S., Broadmeadow, M., Elvira, S., Emberson, L., Gimeno, B.S., Le Thiec, D., Novak, K., Oksanen, E., Schaub, M., Uddling, J., Wilkinson, M., (2007), Risk assessments for forest trees: The performance of the ozone flux versus the AOT concepts, Environmental Pollution, 146, p608-616.
[https://doi.org/10.1016/j.envpol.2006.06.012]

-
Karlsson, P.E., Örlander, G., Langvall, O., Uddling, J., Hjorth, U., Wiklander, K., Areskoug, B., Grenfelt, P., (2006), Negative impact of ozone on the stem basal area increment of mature Norway spruce in south Sweden, Forest Ecology and Management, 232, p146-151.
[https://doi.org/10.1016/j.foreco.2006.05.059]

-
Karnosky, D.F., Zak, D.R., Pregitzer, K.S., Awmack, C.S., Bockheim, J.G., Dickson, R.E., Hendrey, G.R., Host, G.E., King, J.S., Kopper, B.J., Kruger, E.L., Kubiske, M.E., Lindroth, R.L., Mattson, W.J., McDonald, E.P., Noormets, A., Oksanen, E., Parsons, W.F.J., Percy, K.E., Podila, G.K., Riemenschneider, D.E., Sharma, P., Thakur, R., Sôber, A., Sôber, J., Jones, W.S., Anttonen, S., Vapaavuori, E., Mankovska, B., Heilman, W., Isebrands, J.G., (2003), Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project, Functional Ecology, 17, p289-304.
[https://doi.org/10.1046/j.1365-2435.2003.00733.x]

-
Katoh, M., (2004), Classifying tree species in a northern mixed forest using high-resolution IKONOS data, Journal of Forest Research, 9, p7-14.
[https://doi.org/10.1007/s10310-003-0045-z]

- Kohno, Y., Matsumura, H., Ishii, T., Izuta, T., (2005), Establishing critical levels of air pollutants for protecting East Asian vegetation - A challenge, In Plant responses to air pollution and global change, Omasa, K., Nouchi, I., De Kok, L.J., EdsSpringer-Verlag, Tokyo, p243-250.
- Koike, T., Maruyama, Y., (1998), Comparative ecophysiology of the leaf photosynthetic traits in Japanese beech grown in provenances facing the Pacific Ocean and the Sea of Japan, Journal of Phytogeography and Taxonomy, 46, p23-28, (In Japanese with English summary).
-
Massman, W.J., Musselman, R.C., Lefohn, A.S., (2000), A conceptual ozone dose-response model to develop a standard to protect vegetation, Atmospheric Environment, 34, p745-759.
[https://doi.org/10.1016/s1352-2310(99)00395-7]

- Matsuda, K., (2008), Estimation of dry deposition for sulfur and nitrogen compounds in the atmosphere - Updated parameterization of deposition velocity -, Journal of Japan Society for Atmospheric Environment, 43, p332-339, (In Japanese).
-
Matyssek, R., Bahnweg, G., Ceulemans, R., Fabian, P., Grill, D., Hanke, D.E., Kraigher, H., Oβwald, W., Rennenberg, H., Sandermann, H., Tausz, M., Wieser, G., (2007), Synopsis of the CASIROZ case study: Carbon sink strength of Fagus sylvatica L. in a changing environment - Experimental risk assessment of mitigation by chronic ozone impact, Plant Biology, 9, p163-180.
[https://doi.org/10.1055/s-2007-964883]

- Matyssek, R., Sandermann, H., (2003), Impact of ozone on trees: an ecophysiological perspective, In Progress in Botany, 64, Esser, K., Lüttge, U., Beyschlag, W., and Hellwig, F., EdsSpringer-Verlag, Berlin, p349-404.
- Mills, G., Pleijel, H., Büker, P., Braun, S., Emberson, L., Harmens, H., Hayes, F., Simpson, D., Grünhage, L., Karlsson, P.-E., Danielsson, H., Bermejo, V., Fernandez, I.G., (2010), Mapping critical levels for vegetation - Revision undertaken in summer, 2010, to include new flux-based critical levels and response functions for ozone, In Mapping Manual 2004, Spranger, T., EdUNECE Convention on Long-range Transboundary Air Pollution, pIII-4-57.
- Ministry of the Environment, (2004), Comprehensive report on acid deposition survey, Ministry of the Environment, Tokyo, p432, (In Japanese).
- Network Center for EANET, (2009), Data report 2008, Network Center for EANET, http://www.eanet.cc/product.html Accessed 29 June 2011.
-
Ohara, T., Akimoto, H., Kurokawa, J., Horii, N., Yamaji, K., Yan, X., Hatasaka, T., (2007), An Asian emission inventory of anthropogenic emission sources for the period 1980-2020, Atmospheric Chemistry and Physics, 7, p4419-4444.
[https://doi.org/10.5194/acp-7-4419-2007]

-
Paludan-Müller, G., Saxe, H., Leverenz, J.W., (1999), Responses to ozone in 12 provenances of European beech (Fagus sylvatica): genotypic variation and chamber effects on photosynthesis and dry-matter partitioning, New Phytologist, 144, p261-273.
[https://doi.org/10.1046/j.1469-8137.1999.00518.x]

-
Pretzsch, H., Dieler, J., Matyssek, R., Wipfler, P., (2010), Tree and stand growth of mature Norway spruce and European beech under long-term ozone fumigation, Environmental Pollution, 158, p1061-1070.
[https://doi.org/10.1016/j.envpol.2009.07.035]

-
Puxbaum, H., Gregori, M., (1998), Seasonal and annual deposition rates of sulphur, nitrogen and chloride species to an oak forest in north-eastern Austria (Wolkersdorf, 240 m A.S.L), Atmospheric Environment, 32, p3557-3568.
[https://doi.org/10.1016/s1352-2310(98)00073-9]

-
Simpson, D., Ashmore, M.R., Emberson, L., Tuovinen, J.-P., (2007), A comparison of two different approaches for mapping potential ozone damage to vegetation. A model study, Environmental Pollution, 146, p715-725.
[https://doi.org/10.1016/j.envpol.2006.04.013]

-
Sitch, S., Cox, P.M., Collins, W.J., Huntingford, C., (2007), Indirect radiative forcing of climate change through ozone effects on the land-carbon sink, Nature, 448, p791-795.
[https://doi.org/10.1038/nature06059]

- Takagi, K., Ohara, T., (2003), Estimation of ozone impact on plants by damage functions in the Kanto area, Journal of Japan Society for Atmospheric Environment, 38, p205-216.
- Takeda, M., Aihara, K., (2007), Effects of ambient ozone concentrations on Beech (Fagus crenata) seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan, Journal of Japan Society for Atmospheric Environment, 42, p107-117, (In Japanese with English summary).
-
Tanimoto, H., Sawa, Y., Matsueda, H., Uno, I., Ohara, T., Yamaji, K., Kurokawa, J., Yonemura, S., (2005), Significant latitudinal gradient in the surface ozone spring maximum over East Asia, Geophysical Research Letters, 32, L21805.
[https://doi.org/10.1029/2005gl023514]

- Wang, H., Zhou, L., Tang, X., (2006), Ozone concentrations in rural regions of the Yangtze Delta in China, Journal of Atmospheric Chemistry, 54, p255-265.
-
Watanabe, M., Matsuo, N., Yamaguchi, M., Matsumura, H., Kohno, Y., Izuta, T., (2010), Risk assessment of ozone impact on the carbon absorption of Japanese representative conifers, European Journal of Forest Research, 129, p421-430.
[https://doi.org/10.1007/s10342-009-0316-0]

- Watanabe, M., Yamaguchi, M., Iwasaki, M., Matsuo, N., Naba, J., Tabe, C., Matsumura, H., Kohno, Y., Izuta, T., (2006), Effects of ozone and/or nitrogen load on the growth of Larix kaempferi, Pinus densiflora and Cryptomeria japonica seedlings, Journal of Japan Society for Atmospheric Environment, 41, p320-334.
-
Watanabe, M., Yamaguchi, M., Matsumura, H., Kohno, Y., Izuta, T., (2012), Risk assessment of ozone impact on Fagus crenata in Japan: Consideration of atmospheric nitrogen deposition, European Journal of Forest Research.
[https://doi.org/10.1007/s10342-011-0521-5]

- WHO/IPCS, (2004), IPCS Risk Assessment Terminology (Harmonization Project Document No. 1), World Health Organization, Geneva, p117, http://www.inchem.org/documents/harmproj/harmproj/harmproj1.pdf Accessed 25 June 2011.
-
Yamaguchi, M., Watanabe, M., Iwasaki, M., Tabe, C., Matsumura, H., Kohno, Y., Izuta, T., (2007), Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads, Trees, 21, p707-718.
[https://doi.org/10.1007/s00468-007-0163-x]

-
Yamaguchi, M., Watanabe, M., Matsumura, H., Kohno, Y., Izuta, T., (2011), Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species, Asian Journal of Atmospheric Environment, 5, p65-87.
[https://doi.org/10.5572/ajae.2011.5.2.065]

- Yamaguchi, T., Noguchi, I., Eguchi, M., (2010), Ambient ozone concentration around Lake Mashu, Hokkaido, Japan, Transactions of the meeting in Hokkaido branch of the Japanese Forest Society, 58, p123-124, (In Japanese).
-
Yamaji, K., Ohara, T., Uno, I., Kurokawa, J., Pochanart, P., Akimoto, H., (2008), Future prediction of surface ozone over east Asia using Models-3 Community Multiscale Air Quality Modeling System and Regional Emission Inventory in Asia, Journal of Geophysical Research, 113, D08306.
[https://doi.org/10.1029/2007jd008663]

-
Yonekura, T., Yoshidome, M., Watanabe, M., Honda, Y., Ogiwara, I., Izuta, T., (2004), Carry-over effects of ozone and water stress on leaf phenological characteristics and bud frost hardiness of Fagus crenata seedlings, Trees, 18, p581-588.
[https://doi.org/10.1007/s00468-004-0345-8]

- Yoshikado, H., (2004), One possible factor causing recent trend of photochemical oxidants, Journal of Japan Society for Atmospheric Environment, 39, p188-199, (In Japanese with English summary).