Specification of Chemical Properties of Feed Coal and Bottom Ash Collected at a Coal-fired Power Plant
Abstract
In order to offer a better understanding of air pollution of China as well as East Asia we attempted to characterize the chemical properties of the raw coal materials mined in China and their combusted bottom ashes generated from coal fired power plant. To this end, we measured the chemical characteristics of individual bottom ashes and feed coal fragments collected at a coal fired power generator which was operated with the raw coal dug at a coal mine in China. The chemical properties of these two sample types were determined by a synchrotron radiation X-ray fluorescence (SR-XRF) microprobe method. Through an application of such technique, it was possible to draw the 2D elemental maps in and/or on raw coal fragments and fired bottom ashes. The pulverized fine pieces of feed coal mainly consisted of mineral components such as Fe, Ca, Ti, Ca, and Si, while Fe was detected as overwhelming majority. The elemental mass of combusted bottom ash shows strong enrichment of many elements that exist naturally in coal. There were significant variations in chemical properties of ash-to-ash and fragment-to-fragment. Although we were not able to clearly distinguish As and Pb peaks because of the folding in their X-ray energies, these two elements can be used as tracers of coal fire origin.
Keywords:
Coal, Bottom ash, Coal-fired power generation, X-ray fluorescence, Trace element1. INTRODUCTION
China’s energy demand and consumption has risen dramatically due to its rapid industrialization. Its energy demand has largely been met with coal-fired power plants which correspond to over 80% of domestic power generation capacity (China’s Development Research Center of the State Council).
Coal-fired power stations produce large quantities of waste ash and fly ash. These coal plants worsen air quality and creates acid rain which then hurts soil quality and food safety. Fly ash contains many trace elements of environmental concern. These harmful byproducts of coal combustion of China should be a grave pollution problem in East Asia. The leaching of trace elements in fly ash must therefore be monitored to reduse its hazardous impacts.
Particulate matters would be expected to be affected by long-range transport (Danalatos and Glavas, 1999; Luria et al., 1996; Duce et al., 1980). Over the past few decades, enormous efforts have been directed to the study of pollutants emitted from Chinese continent and their impacts on East Asia and Pacific Basin (Ma et al., 2004; Wang et al., 2000; Duce et al., 1980). In the case of sulfate, the calculated residence time is generally estimated between 2.7 and 7.2 days (Seinfeld and Pandis, 1998). Long-range transport is important if typical lifetimes for the species of interest exceed several days. Parungo et al. (1994) also suggested that aerosol black carbon originated from the widespread fossil fuel combustion in China should have long residence times in the free troposphere to be transported over great distance. Furthermore, Tazaki (2009) suggested that the acid rain, sulfur materials, and mineral dust deposited on the surfaces of black pine trees of Hekura-jima, which is located in the sea between Korea and Japan, were originated from the neighboring countries rather than from local sources.
It is suggested that the incorporation of anthropogenic fine particles (including fly ash) into dust storm particles may occur at the industrial areas in China which can then be subject to long-range transport to the receptor area in East Asia as well as the Pacific Ocean. From the results of a single particle analysis for the particles collected at Fukuoka, Japan, Ma and Choi (2007) reported that the chemically mixed particles (coarse crustal particles mixed with fine artificial ones; coarse crustal and sea-salt particles mixed with fine artificial ones) were able to account for 69.2 and 77.2% of the particle with 5.1, and 7.7 μm size, respectively.
Information concerning the nature of fly ash emitted from coal fired power station is prerequisite to the diagnosis of an environmental impact of coal combustion residues that may exert in China as well as East Asia. However, only few studies have been made to describe the nature and composition of fly ash emitted coal-fired power stations located in East Asia (Tsuji et al., 2009a, b).
The properties of individual fresh fly ash can be chemically altered by several processes like gas-to-particle transformation, coagulation, and growth processes of particles during long-rage transport. Hence, to account for the initial properties of fly ash particle, the data derived by single particle analysis can be incorporated effectively with the assessment of its source inventory.
In an effort to precisely describe the nature of raw feed coal and fired fly ash, an intensive measurement was conducted using the world’s largest third-generation synchrotron radiation facility. This facility is well-known as the most powerful synchrotron radiation for the analysis of infinitesimal trace elements from various samples including individual fly ash particles.
Bottom ash is the coarse, solid mineral residue that results from the burning of coal. The individual particles are much larger than fly ash particles and fall down through the air flow to the bottom of the coal burning furnace. This bottom ash has a similar chemical composition to fly ash (McKerall et al., 1982). Here, we report the chemical nature of the individual raw feed coal and bottom ashes produced from the Chinese coal products during the operation of the Taean coal fired thermal power plant in Korea.
2. EXPERIMENTAL METHODS
2. 1 Description of Facility
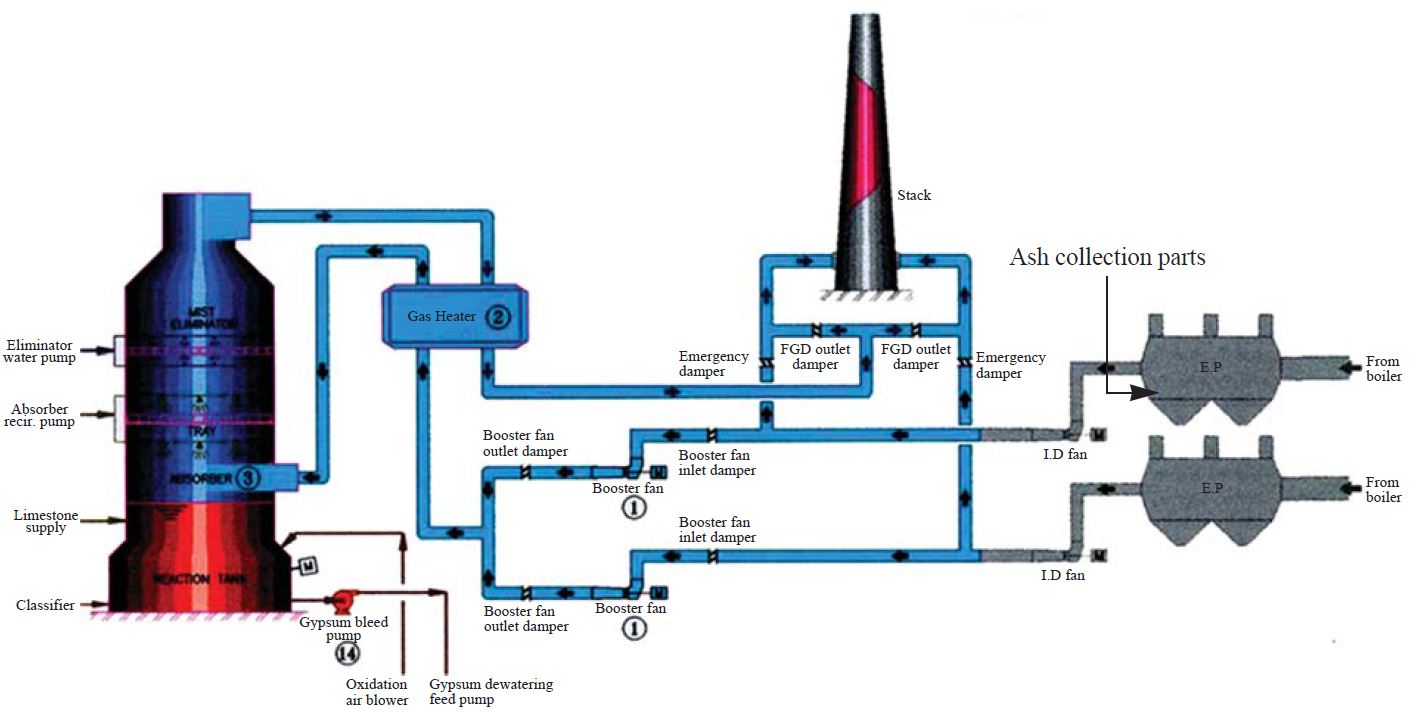
In Korea, almost all coal used for power generation are imported from Australia, Indonesia, India, and China. The Taean thermal power generation station (36.17°N; 126.13°E) is operated with imported bituminous coal. This coal was mined at Guizhou Province located in southwest China. The Taean thermal power generation station (total of 8 units) is the biggest branch of the Korea Western Power Co., Ltd. (WP), which takes up approximately 13% of the national electric power generating capacity which consists of thermal, combined cycle, and pumped storage power plants. The Taean thermal power generation station produces electricity with a capacity of 4,000MW, accountig for approximately 45% of WP’s total capacity (Korea Western Power Co., Ltd.). Fig. 1 illustrates a flow diagram of power generation in the target facility. In each operation unit, the coal is burned in a two-pass boiler.
2. 2 Sample Collection
In this study, the pulverized feed coal was collected before being supplied to the combustion system. To obtain representative coal samples, three individual points were selected from coal supplying system. The collected samples were then mixed randomly. The boiler of each operation unit is built as the conventional balanced draught system, with forced draught and primary air fans from the top of the boiler-house in which flue gases are drawn by the draught fan in the boiler. Each boiler has two sets of electrostatic precipitators (EP) (see Fig. 1) which removes particulates mixed with flue gas. The fly ash is extracted from EP hoppers of each respective boiler through hydraulic exhausters up to a collector tank by means of water pumps for the separatioin of the fly ash. Dry ash was collected at hopper of the upper EP in Fig. 1.
2. 3 Chemical Analysis
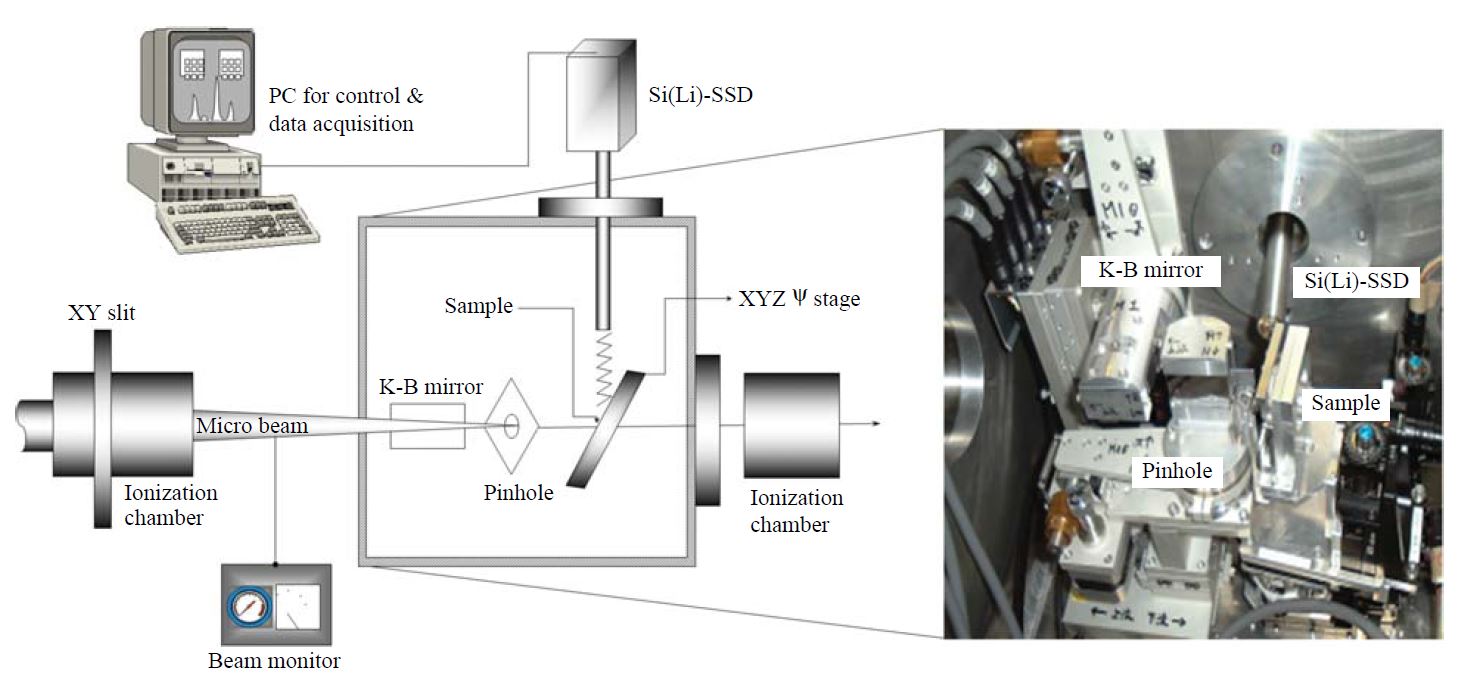
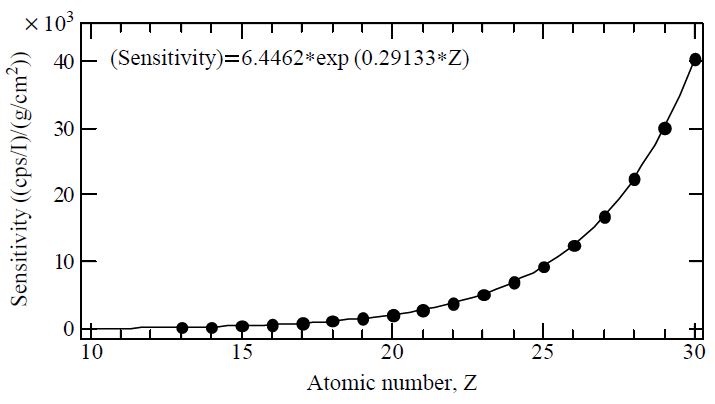
As bulk analytical methods are generally done by collecting samples of many fragments and particles on a sample holder, such techniques are often restricted to explain the particle-to-particle (or fragment-to-fragment) composition variations. In this study, the ultra trace elements in the individual raw feed coal fragments and fired bottom ashes were identified by an X-ray microprobe system equipped at the Super Photon ring 8 GeV (SPring-8), BL-37XU. Through an application of this micro-analytical technique based on the X-ray fluorescence (XRF) method, multiple elements were successfully analyzed at femtogram level sensitivity. When coal fragments and fired bottom ashes form a cluster, such file up can obscure the chemical determination of particle-to-particle (or fragment-to-fragment) variations. Therefore, raw feed coal fragments and fired bottom ashes were deposited independently without overloading the substrate on the non-hole Nuclepore® filter (25 mm diameter). This sample was then placed on the XY scanning stage in a vacuum chamber (Fig. 2). The sample areas (500-1,000 μm2 each time) were then selected randomly and scanned by the microbeam. The takeoff angle of 10°was used for the measurement of X-ray fluorescence, and the intensity of the incident X-rays was monitored by an ionization chamber. The XRF elemental image of sample can be obtained via the scanning processes. The point analysis for individual particles (fragments) was carried out subsequently. The fluorescence X-rays were recorded with a Si(Li) detector placed in the electron orbit plane of the storage ring. The detector was mounted at 90°to the incident X-rays to minimize the the scattering background. Fig. 3 shows experimental calibration (sensitivity [(count/beam intensity)/(g/cm2)]) curve for standards. Details of the calibration of the X-ray microprobe system to yield quantitative measures of elemental mass in individual particles including the effects of the self-absorption are described elsewhere (Hayakawa et al., 2001). By means of this XRF analytical technique, a total of 122 individual feed coal powders and 105 combusted bottom ashes were analyzed in this research.
3. RESULTS AND DISCUSSION
3. 1 Feed Coal Properties
The content, distribution, and behavior of elements released during and after combustion depend largely on the counterpart properties of the raw feed coal. Coal contains a large amount of various elements including toxic metals and metalloids. These elements cannot be destroyed during the combustion processes of coal, although released in various throughputs such as fly ash, bottom ash, wastes from gas scrubber units, and gaseous emissions to the atmosphere (Brigden et al., 2002). Thus, information on the chemical composition of feed coal is a valuable due to assess its impact on local ecosystem as well as more long-range area.
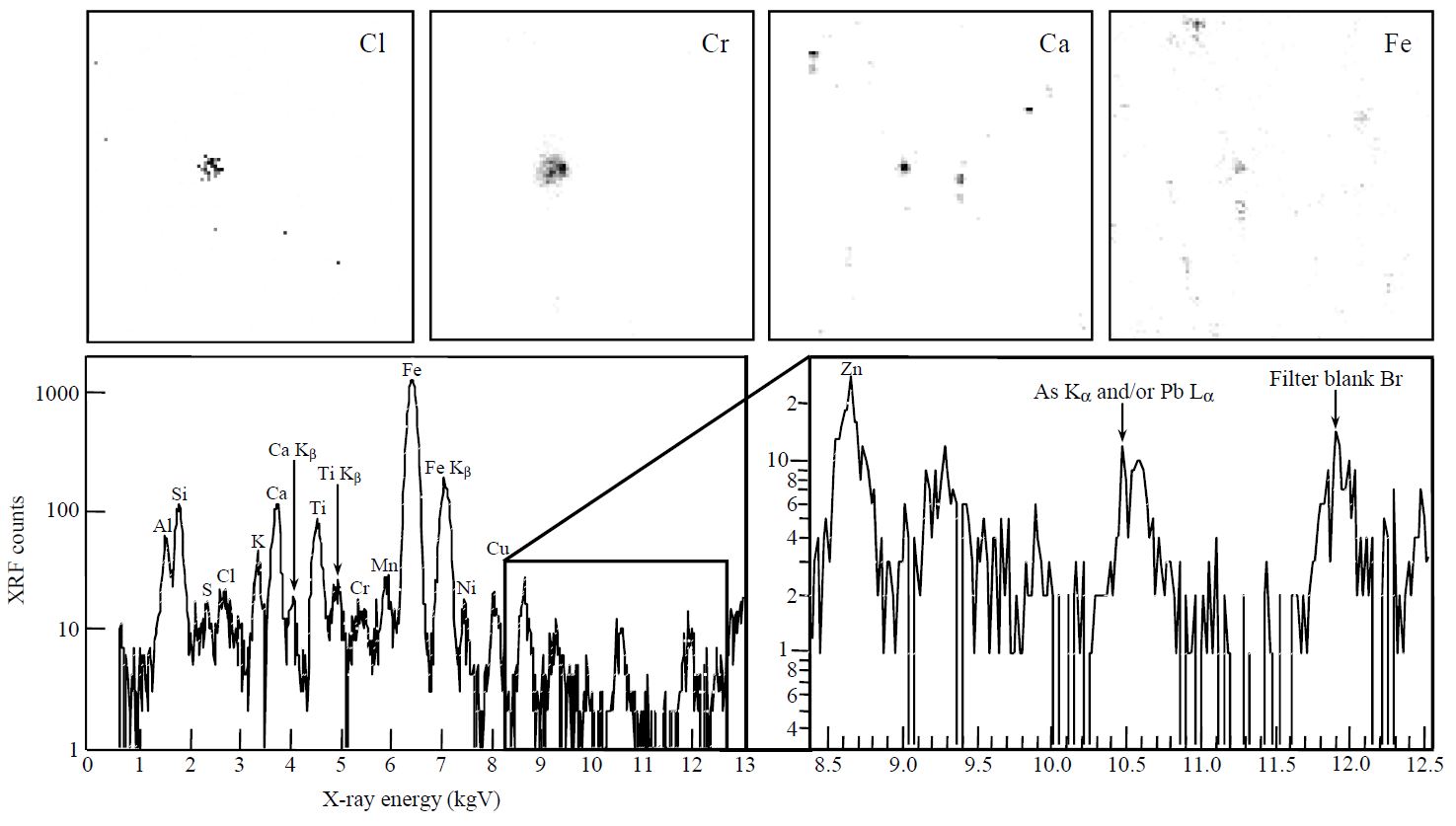
An example of XRF elemental maps of five characteristic elements (S, Ca, Cl, Fe, and Pb) drawn individually on separated raw coal fragments is illustrated in Fig. 4. Each elemental map was drawn on the 500×500 μm2 scanning area of microbeam. As shown in Fig. 4, it was possible to clearly reconstruct elemental maps for individual fragment of feed coal. From XRF elemental maps, it can be visually confirmed that Fe is present consistantly as the major component. In contrast, S, Ca, Cl, and Pb are partially concentrated on few coal granules. As this dissimilarity can vary with the irradiated points, the chemical composition of individual coal fragments is unlikely homogeneous. This lack of chemical uniformity among individual coal fragments is probably caused by the fact that the chemically heterogeneous fine coal pieces were formed via crushing processes of a lump of raw coal before feeding to combustion system. The chemically unevenness of coal pieces may be able to be chemically inhomogeneous fly ash.
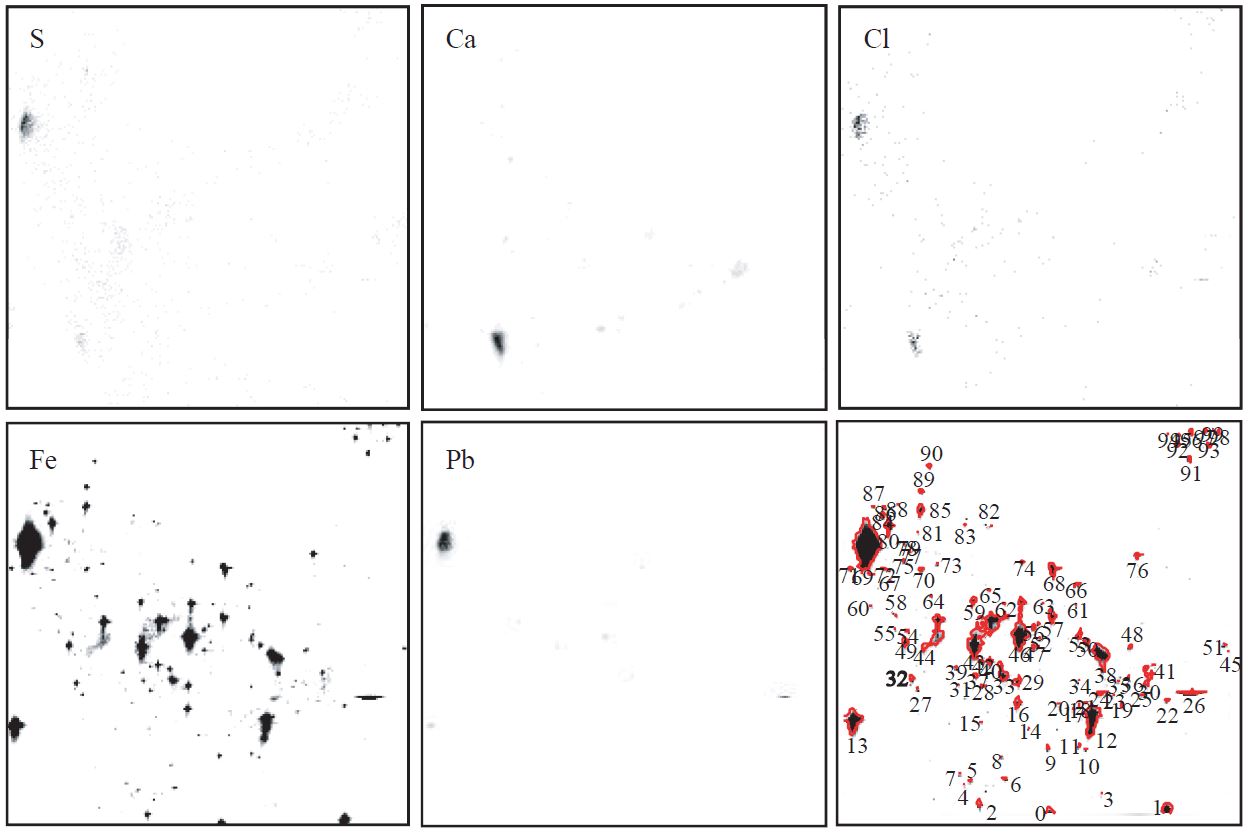
XRF images of Pb, Fe, Cl, Ca, and S, and the location of granulated raw coal fragments in a full scanning area with 500×500 μm2.
Fig. 5 shows the relative intensity of XRF counts for five elements in raw coal powders. Five characteristic elements in coal fragments, especially Fe, Ca, and Pb, appear to experience significant piece-to-piece variation. This inhomogenity of XRF counts among broken pieces can be probably derived from the dissimilarities of crushed coal sizes as well as chemical compositions of original coal samples.
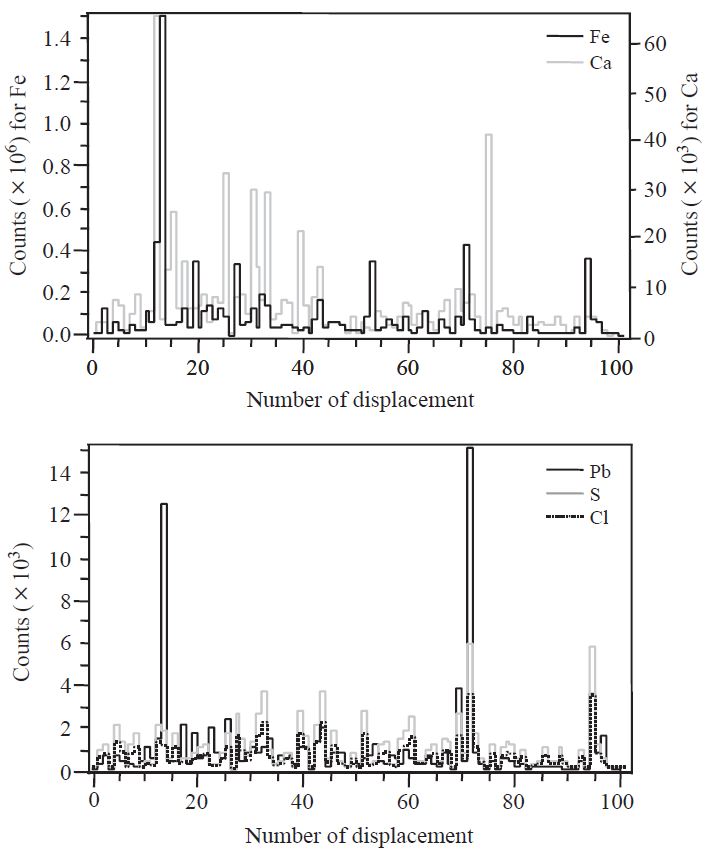
Relative intensity of XRF counts for five elements in raw coal powder mined in China. Number of displacement means the numbers of individual coal fragments irradiated by an X-ray microbeam.
The XRF spectrum drawn at point number 32 in the full scanning area of Fig. 4 is also drawn in Fig. 6. This XRF spectrum can be drawn by counting XRF counts of each element during the point analysis. In line with general expectation, there is a tendency towards higher XRF counts for soil-derived elements (e.s., Fe, Ca, Ti, Ca, and Si). Elsewhere, the magnified XRF spectrum in Fig. 6 also shows a meaningful peak of As (10.53 keV of Kα) and/or Pb (10.55 keV of Lα). Although it was not possible to discriminate between As (Kα) and Pb (Lα) in this study, the existence of both elements, which has been identified as hazardous air pollutants, was already found in raw coal. Liu et al. (2002) reported the health effects of arsenic exposure from burning coal which was burned inside the home in open pits for daily cooking and crop drying in China. Miller et al. (1998) also detected Pb (12.8 ppm) in raw coal through the emissions testing in the pilot-scale combustor.
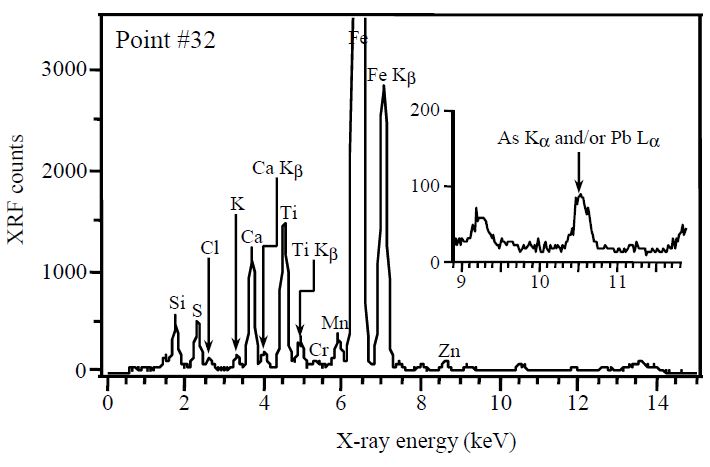
XRF spectra drawn by irradiating an X-ray microbeam on a coal fragment (point # 32 at bottom right of Fig. 3).
The mass (fg) for major 17 elements in the individual bottom ash and feed coal fragments is statistically summarized in Table 1. The element ratios of most metals to Si for the samples of both present study and literature (Ma et al., 2005, 2004) are also listed in Table 1. Here we described preferentially the chemical nature of feed raw coal. The measured Fe mass is seen to range from 8.74 to 29,358 fg (average 1,353 fg); the later value is the highest average mass. This Fe is probably derived from the mineral compositions such as magnetite (FeFe3O4), magnesioferrite (MgFe2O4), and pyrite (FeS2). Mineral components like [1] Ca (possible sources; calcite(CaCO3), apatite (Ca5(PO3)3F), and anhydrite (CaSO4)), [2] Si (possible sources; quartz (SiO2) and kaolinite (Al2Si2O5(OH)4)), and [3] Ti (possible source; perovskite (CaTiO3)) are followed. They are in the range of 5.79 to 8,312 fg (average 763 fg), 7.75 to 4,814 fg (average 602 fg), and 2.34 to 2,559 fg (average 314 fg), respectively. Likewise, sulfur also shows the significant mass (~1,892 fg) with average 278 fg.
3. 2 Bottom Ashes Properties
The chemical composition of fly ashes as one of the end byproducts after coal combustion is dependent on both the mineral composition of coal and boiler operation conditions (Miller and Linak, 2002).
Fig. 7 shows an example of XRF elemental maps for four kinds of elements and the XRF spectra of a bottom ash collected at the Taean coal-fired power generator. According to the elemental distribution displayed in Fig. 7, it is most likely that several elements coexist in fly ash as the chemical mixing state. The minor trace elements (Zn, As (and/or Pb), and Br) also appeared in compliance with the partially magnified X-ray energy range from 8.5 to 12.5 keV. As shown in Fig. 7, many elemental components including various mineral and trace elements have still relatively distinct XRF peaks. It is expected that the chemical composition of fly ashes is dependent on both the mineral composition of coal and the combustion conditions of boilers. Here we have views on the XRF peak of As and/or Pb formed around 10.5 keV. It is not easy to clearly identify this peak, as mentioned earlier, because the energy range of As is in contiguity with that of Pb. Hence, we interpreted this XRF peak by assuming the coexistence of both elements in bottom ash. If this peak is really representative of Pb (Lα), changes need be made to the existing interpretation of Pb source. Alhough it was banned to run leaded gasoline-powered vehicles, this Pb with Br in urban ambient particles has still been considered to come from automobile source as fallout of engine exhaust (EPA, 1998). The result of this study suggests that one has to carefully estimate the source of Pb in atmospheric aerosols because coal combustion can also be a good source of Pb.
As is typically found in uncontaminated soil at concentration up to 50 mg kg-1 (Alloway, 1990). However, if this obscure peak is that of As, the ratio of As to other elements (e.g., As/Fe) can be a valuable tool to estimate the long-range transport of anthropogenic pollutants transported from China to East Asia. Note that the concentration of As in the fly ash sample from the Mae Moh plant in Thai was over three times the background soil level (Brigden and Santillo, 2002).
In order to lower the level of pollutnats contained in fly ash, it is beneficial to understand the alternation of coal composition between the time of mining and deposition of the fly ash. However, it is difficult to evaluate the relationship between feed coal and fly ash because power plants commonly use blends of coals or mixtuers of coals and other materials (for example, tires or biomass) to run their boilers. As shown in Table 1, the elements of bottom ash, which is a coal ash recovered in an EP, also contains the main mineral elements such as Si, Al, Ca, and Fe as well as small quantities of heavy elements including Cr, Sc, V, and Mn.
The relative elemental ratios summarized in Table 1 can be used as a simple barometer to assess the transformation of dust particles during long-range transport and other aging processes. Bottom ash from EP is also characterized by higher Cl ratio to Si (0.484) than that of feed coal (0.177). Bottom ash also contains much lower ratio of Fe to Si (0.226) than that of feed raw coal (2.248). As references of these fly ash data, we make the comparison of Cl/Si ratios of individual particles among bottom ash, desert sand in China, and two background sites of Korea and Japan. The Cl/Si ratio of bottom ash (0.455) in this study is quite high compared to that of desert sands (0.058). Therefore, although such as automobiles and municipal incinerators can also be considered as the sources of ambient Cl in the receptor area of Asian dust, one cannot rule out the possibility that Cl is driven from coal consumption (e.g., coal-fired power plant).
Relatively high Cl/Si ratios in individual Asian dust particles collected at both background sites of Jeju, Korea (Cl/Si 2.707) and Tango, Japan (Cl/Si 0.807), especially Jeju, Korea, compared to those of bottom ash (Cl/Si 0.455) and desert sand (Cl/Si 0.058) are found in Table 1. The Cl enrichment of coarse fraction particles can be apparently explained by the fact that Asian dust storm particles were absorbed or coalesced with particles containing sea-salt during the long-range transport (Wang and Guanghua, 1996). The higher Cl/Si ratio in particles at Jeju site than that in particles at Tango might be relevance to the dissimilarity of particle size (i.g., large size (>1.2 μm) at Jeju and small size (1.17 μm) at Tango).
If plant and animal are exposed to high concentrations of trace elements with high toxicity, significant bioaccumulation can take place (Kimbrough et al., 1999). The relative ratios of several toxic elements to Cr in fly ashes (collected from various ash producers) are presented in Table 2. This result shows differences in the relative compositions among feed coals combusted at each different power stations. These dissimilarities of elemental compositions among fly ash sources can also be explained by the control facilities such as wet scrubber. Kimbrough et al. (1999) reported that the higher concentration of toxic metals in the fly ash from the Mae Moh power generator might result from the use of ionizing wet scrubbers at that facility which could have facilitated the removal of those elements (from gaseous emissions) into solid waste streams. As compared with the previous studies which were carried out with bulk sample, relatively low normalized values for Mn, Ni, and Zn are shown in this study. The reason might be that the mass of Cr (1.42 fg) determined in the present single particle study was overwhelmingly (about 5-30 times) higher than those of other trace metals (Mn 0.27 fg, Ni 0.08 fg, and Zn 0.05 fg). The similar mass concentration of these elements (Cr 5,555 mg/kg, Mn 0.001 mg/kg, Ni 2,234 mg/kg, and Zn 937 mg/kg) in ash which was bulkily collected from the coal fired boiler in Guizhou, China was also reported (He et al., 1998). Therefore this predominantly high Cr concentration might be a chemical peculiarity of the ash produced from combustion of Chinese coal material. This normalization procedure for several toxic metals enables us not only to understand the chemical disequality of fly ashes among different coal power stations but also to estimate their ambient source profiles.
4. CONCLUSIONS
Being the dominant power generation (i.e., more than 70 percent of electricity) in China, coal combustion is regarded as a significant atmospheric polluter as well as a potential environmental problem in East Asia. Thus, understanding the chemical nature of raw coal and its byproducts is essential to cope with air pollution problem in both China and East Asia. In this study, the chemical characteristics of raw feed coal and burned fly ash were analyzed with the aid of the 2D elemental map. In addition, relative elemental mass was also examined by using the world’s largest third-generation synchrotron radiation facility. Many elemental components (including various mineral and trace elements) were detected from both samples. It was also found that individual bottom ashes and feed coal fragments were chemically inhomogeneous. As and/or Pb detected from individual bottom ashes can be one of the most useful ways to estimate the long-range transport of artificial pollutants and their source. In our future study, in order to fully assess the relationship between feed coal and fly ash, we are planning to match fly ash from power plants with their single-sourced coal. In addition to this, numerous samples must be analyzed in order to elucidate the properties of the most representative fly ash samples.
Acknowledgments
This study was supported in part by funds from the Grant-in-Aid for Scientific Research on Priority Areas under Grant No. 14048212 and 14048213 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Also this study was partly carried out under the aid of the program “Establishment of COE on Sustainable-Energy System”. The synchrotron radiation experiments were performed at the SPring-8 with approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2002B0395-NOS-np, 2002A4029-LM-np).
The authors wish to express thanks to all the members of SPring-8, BL-37XU.
References
- Alloway, B.J., (1990), Heavy metals in soils, John Wiley and Sons, Inc., New York, ISBN 0470215984.
- Brigden, K., Santillo, D., (2002), Heavy metal and metalloid content of fly ash collected rom the Sual, Mauban and Masinloc coal-fired power plants in the Philippines, 2002, Technical note of Greeenpeace Research Laboratory, UK, p1-23.
- Brigden, K., Santillo, D., Stringer, R., (2002), Hazardous emissions from Thai coal-fired power plants: Toxic and potentially toxic elements in fly ashes collected from the Mae Moh nad Thai Petrochemical Industry coal-fired power plants in Thailand, 2002, Technical note of Greenpeace Research Laboratory, UK, p1-26.
- China’s Development Research Center of the State Council, (2010), Industrialization and energy intensity, http://www.drcnet.com.cn/Drcnet.channel.web/english/index.aspx.
-
Danalatos, D., Glavas, S., (1999), Gas phase nitric acid, ammonia and related particulate matter at a Mediterranean coastal site, Patras, Greece, Atmospheric Environment, 33, p3417-3425.
[https://doi.org/10.1016/s1352-2310(98)00342-2]

-
Duce, R.A., Unni, C.K., Ray, B.J., Prospero, J.M., Merrill, J.T., (1980), Long-range atmospheric transport of soil dust from Asia to the topical North Pacific: temporal variability, Science, 209, p1522-1524.
[https://doi.org/10.1126/science.209.4464.1522]

- EPA, (1998), Locating and estimating air emissions from sources of lead and lead Compounds, EPA-454/R-98-006, p7-8.
- Hayakawa, S., Ikuta, N., Suzuki, M., Wakatsuki, M., Hirokawa, T., (2001), Generation of an X-ray microbeam for spectromicroscopy at SPring-8 BL39XU. Search Results, Journal of Synchrotron Radiation, 8, p328-330.
- He, J., Tan, H., Sommar, J., Xiao, Z., Lindqvist, O., (1998), Mercury pollution in a mining area Guizhou, China: Fluxes over contaminated surfaces and concentrations in air, biological and geological samples, Toxicity Environmental Chemistry, 67, p225-236.
-
Kimbrough, D.E., Cohen, Y., Winer, A.M., Creelman, L., Mabuni, C., (1999), A critical assessment of chromium in the environment, Critical Reviews in Environmental Science and Technology, 29(1), p1-46.
[https://doi.org/10.1080/10643389991259164]

- Korea Western Power Co., Ltd., (2008), 2008 Sustainability Report, http://www.westernpower.co.kr.
- Liu, J., Zheng, B., Aposhian, V., Zhou, Y., Chen, M.L., Zhang, A., Waalkes, M.P., (2002), Chronic arsenic poisoning from burning high-arsenic-containing coal in Guizhou, China. Search Results, Environmental Health Perspectives, 110, p119-122.
-
Llorens, J.F., Fermandez-Turiel, J.J., Querol, X., (2001), The fate of trace elements in a large coal-fired power plant, Environmental Geology, 40(4-5), p409-416.
[https://doi.org/10.1007/s002540000191]

- Luria, M., Peleg, M., Sharf, G., Tov-Alper, D.S., Spitz, N., BenAmi, Y., Gawii, Z., Lifschits, B., Yitzchake, A., Seter, I., (1996), Atmospheric sulfur over east Mediterranean region, Journal of Geophysical Research, 101, p25917-25930.
- Ma, C.-J., Choi, K.-C., (2007), A combination of bulk and single particle analyses for Asian dust study, Water Air, and Soil Pollution, 183(1), p3-13.
-
Ma, C.-J., Kasahara, M., Tohno, S., Kim, K.-H., (2008), Physicochemical properties of Asian dust sources, Asian Journal of Atmospheric Environment, 2(1), p26-33.
[https://doi.org/10.5572/ajae.2008.2.1.026]

-
Ma, C.-J., Tohno, S., Kasahara, M., Hayakawa, S., (2004), Properties of individual Asian dust storm particles collected at Kosan, Korea during ACE-Asia, Atmospheric Environment, 38, p1133-1143.
[https://doi.org/10.1016/j.atmosenv.2003.11.020]

- Ma, C.-J., Tohno, S., Kasahara, M., Hayakawa, S., (2005), A case study of the size-resolved individual particles collected at ground-based site on west coast of Japan during Asian dust storm event, Atmospheric Environment, 39, p739-747.
- McKerall, W.C., Ledbetter, W.B., Teague, D.J., (1982), Analysis of fly ashes produced in Texas, Texas Transportation Institute, Research Report No. 240-1, Texas A&M University, College Station, Texas.
- Miller, C.A., Linak, W.P., (2002), Primary particles generated by the combustion of heavy fuel oil and coal, Review of Research Results from EPA’s National Risk Manage, Res. Lab., EPA-600/R-02-093, p31-35.
- Miller, S.F., Wincek, R.T., Miller, B.G., Scaroni, A.W., (1998, Trace element emissions when firing pulverized coal in a pilot-scale combustion facility, 23rd International Technical Conference on Coal Utilization & Fuel Systems, Florida, US, Mar, 9-13).
-
Parungo, F., Nagamoto, C., Zhou, M.Y., Hansen, A.D.A., Harris, J., (1994), Aeolian transport of aerosol balck carbon from China to the ocean, Atmospheric Environment, 28(20), p3251-3260.
[https://doi.org/10.1016/1352-2310(94)00164-g]

- Seinfeld, J.H., Pandis, S.N., (1998), Atmospheric Chemistry and Physics, John Wiley & Sons, Inc., p51-66.
- Tazaki, K., (2009), Aerosol particle capture by microbial mats at Solitary Islands in the Sea of Japan, Journal of the Clay Science Society of Japan, 48(1), p27-42.
-
Tsuji, H., Ikeda, M., Shirai, H., Kotsuji, T., (2009a), Comparison of particle densities and blaines of fly ashes generated in a pulverized coal combustion test facility and in pulverized coal-fired power stations, Transactions of the Japan Society of Mechanical Engineers, 75(756), p1718-1720.
[https://doi.org/10.1299/kikaib.75.756_1718]

- Tsuji, H., Ikeda, M., Shirai, H., Kotsuji, T., (2009b), An experimental study on effects of combustion conditions and coal properties on fundamental properties of fly ash by use of a pulverized coal combustion test facility (Thermal Engineering), Transactions of the Japan Society of Mechanical Engineers, 75(752), p831-838.
-
Wang, X., Guanghua, Z., (1996), Some characteristics of the aerosol in Beijing, International Journal of PIXE, 6, p361-365.
[https://doi.org/10.1142/s0129083596000387]

-
Wang, Z., Ueda, H., Huang, M., (2000), A deflation module for use in modeling long-range transport of yellow sand over East Asia, Journal of Geophysical Research, 105, p26947-26960.
[https://doi.org/10.1029/2000jd900370]