Changes in Concentration Levels of Polycyclic Aromatic Compounds Associated with Airborne Particulate Matter in Downtown Tokyo after Introducing Government Diesel Vehicle Controls
Abstract
The effectiveness of the government regulation on tail-pipe emission for diesel vehicles issued in 2003 in Tokyo was evaluated in this study. Variations in annual average concentrations of polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs associated with airborne particulate matter were investigated in connection with the variation in airborne elemental carbon (EC) concentration in downtown Tokyo, Japan in 2006-2007 and in 1997-1998. The annual average concentrations of EC, seven different PAHs, and 1-nitropyrene were found to have decreased significantly from 1997-1998 to 2006-2007. The most prominent decrease in atmospheric concentration was observed for 1-nitropyrene, which is a representative nitro-PAH originating from diesel vehicles. This indicated that the government control has worked to considerably reduce both atmospheric mutagens and airborne particulate matter. In contrast, the concentrations of two nitro-PAHs, 2-nitrofluoranthene and 2-nitropyrene, remained the same. These nitro-PAHs are known to be formed by atmospheric nitration of their parent PAHs, and this result suggested factors other than the concentration of parent PAHs and NO2 affects the degree of atmospheric formation of nitro-PAHs.
Keywords:
PAHs, Nitro-PAHs, Airborne particulate matter, Diesel vehicle, Elemental carbon1. INTRODUCTION
Air pollution caused by vehicle emissions has been of great concern in terms of the increasing number of lung cancer patients. Lung cancer is currently the leading cause of death in Japan. Particulate matter (PM) is regarded as an extremely important pollutant especially in urban areas since it contains various mutagenic or carcinogenic polycyclic aromatic compounds (PACs), some of which are found both in diesel engines and in airborne PM (Kakimoto et al., 2002; Hayakawa et al., 1995; Murahashi et al., 1995). Among the mutagenic PACs, two groups are well known. One group is the polycyclic aromatic hydrocarbons (PAHs), such as benzo[a]pyrene, which exhibit mutagenicity expressed by metabolic activation (Nisbet and LaGoy, 1992). PAHs are formed during combustion of organic compounds and a considerable fraction of them, especially those with high molecular weight, aggregate on PM generated during the combustion processes of organic compounds due to their low vapor pressure (Mastral and Callen, 2000). The other PAC group is the nitro-PAHs, which show direct-acting and stronger mutagenicity (Rosenkranz and Mermelstein, 1983). Some nitro-PAHs are also believed to be formed in the combustion process especially at high temperature, such as in diesel engines because an elevated concentration of nitrogen oxides is attained under such conditions (Tang et al., 2005). Such nitro-PAHs, represented by 1-nitropyrene (1-NP), have been identified and determined both in airborne PM (Tang et al., 2005; Kakimoto et al., 2002; Hayakawa et al., 1995; Murahashi et al., 1995) and diesel exhaust particulate (DEP) (Schuetzle, 1983). There are also some nitro-PAHs that are believed to be formed via atmospheric reactions of parent PAHs with nitrogen oxides (Phousongphouang and Arey, 2003; Ishii et al., 2001; Enya et al., 1997; Arey et al., 1986; Zielinska et al., 1986; Pitts et al., 1978). For example, it is believed that 2-nitrofluoranthene (2-NF) and 2-nitropyrene (2-NP) are produced in the atmosphere via the reactions of fluoranthene or pyrene with NO2, which are initiated by OH radicals in the daytime or by NO3 radicals in the nighttime (Arey et al., 1986; Zielinska et al., 1986).
In 2003, additional regulations to those already in place from the national government were enforced by the Tokyo metropolitan government on emissions for diesel vehicles used in Tokyo. As a result, the diesel vehicles not meeting this standard were to be replaced with newer low-emission models or have PM reduction devices installed, such as a diesel particulate filter (DPF) (Yokota, 2007; Tokyo Metropolitan Government, 2006). In recent years, the concentration of suspended particulate matter (SPM, particles with a 100% cut-off aerodynamic diameter <10 μm) has been decreasing in downtown Tokyo according to government environmental monitoring data. However, there are limited reports on the change in concentration levels of individual pollutants associated with airborne PM since implementation of the government diesel vehicle controls. Above all, the change in mutagenic PAC concentration is of great importance from a public health point of view.
In this study, the variations of annual concentration levels of PAHs and nitro-PAHs associated with airborne PM in downtown Tokyo before and after the regulation became effective (i.e. 1997-2006) were investigated. Airborne elemental carbon (EC) was also investigated, as it is an excellent indicator of diesel emissions. These studies would help reveal the effectiveness of the regulation in reducing atmospheric mutagenic PACs. A comparison with the concentration changes at neighboring suburban sites was also made with respect to the same pollutants for further evaluation of the effectiveness of the regulation.
2. EXPERIMENTAL
2. 1 Sampling of Airborne PM
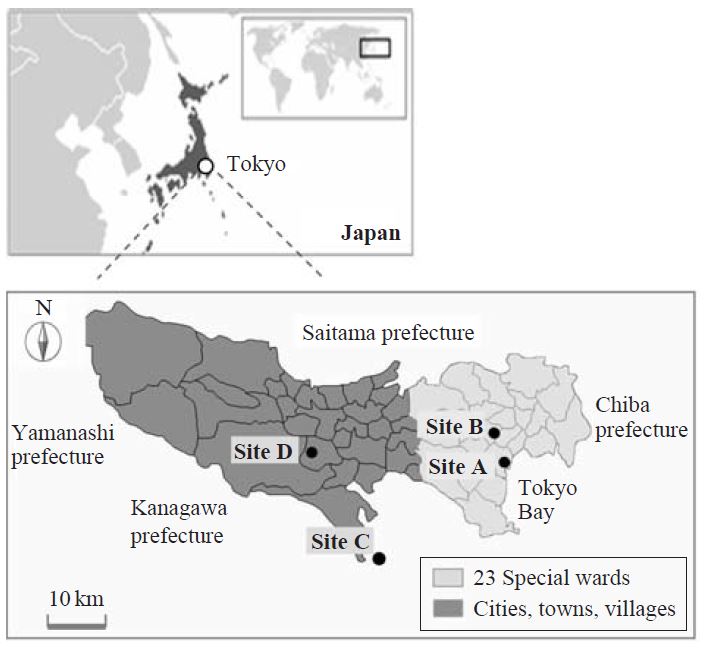
Airborne PM was collected on a quartz fiber filter (8×10 inch2, Pallflex Product, 2500QATUP) using a high-volume air sampler (HV-1000F, Sibata Co.) equipped with an impactor stage to eliminate any particle larger than 10 μm in aerodynamic diameter. The flow rate was 0.5 or 1.1 m3 min-1 and samples were collected on the rooftop of the building at each site (A, B, C and D in Fig. 1). After collection, the filter samples were dried at room temperature, and weighed to determine the concentration of the PM. All filters were then stored at -30°C until being submitted for analysis. Sites A and B were located in typical urban areas that included busy roads with a traffic density of 40,000-80,000 cars per day. Sites C and D were located in representative suburban areas of Tokyo. Twenty-four-hour sampling programs were carried out on 4-6 days in each month of May, September, and January in 1997-1998 (site A) and in 2006-2007 (site B), in January 2001 (sites A and C), and in October 2005 (sites B and D). The change in concentration levels of SPM, EC and PACs from before to after the regulation became effective were analyzed by comparing the concentrations in 1997-1998 (site A) and 2006-2007 (site B). Although comparing the concentrations at different sampling sites is not an ideal way to evaluate the effectiveness of the newly issued regulation on emission from diesel vehicle in Tokyo, the air quality at the two sampling sites in this study is expected to be almost the same for the following reasons. First, as described later (Results and Discussion), SPM concentrations at the two sites are almost the same during the last ten years (Fig. 1). Second, both sites can be regarded as urban background sites that are not significantly influenced by direct emissions from the traffic roads because the actual points of the sampling were more than 100m away from the traffic roadside to avoid the exclusive impact from particular traffic roads. Finally, as described later (Results and Discussion), no significant difference in the concentration of PACs was observed between the urban and suburban sites (e.g. sites A and C) which are more than 20 km distant from each other (Fig. 5), indicating that the concentration level of PACs at site A and B only approximately 5 km distant from each other should also be the same. Considering these facts, the differences in concentrations of these compounds from 1997-1998 (site A) to 2006-2007 (site B) are suitable to evaluate changes in the air quality induced by the regulation in Tokyo although it’s the second best way.
2. 2 Materials
Benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF), and indeno[1,2,3-cd]pyrene (IP) were obtained from Wako Pure Chemical Industries. Pyrene (PY), benzo[ghi]perylene (BghiP), and perylene (PER) were obtained from Aldrich Chemical Co. Fluoranthene (FL) and 1-nitropyrene (1-NP) were obtained from Tokyo Kasei Kogyo. 2-nitrofluoranthene (2-NF) and 2-nitropyrene (2-NP) were obtained from Chiron AS. All the chemicals were used without further purification.
2. 3 Analysis of PAHs and Nitro-PAHs
A section of each filter was cut into small pieces and put in a flask followed by addition of an internal standard. Then both PAHs and nitro-PAHs were extracted ultrasonically for 20 min twice with dichloromethane (DCM) and the extract solution was filtered to remove solid material. The filtrate was concentrated to dryness under a flow of N2. For PAH analysis, the residue was dissolved in 500 μL of DCM and the solution was cleaned by solid phase extraction (SPE) with silica gel as the solid phase (Discovery SPE DSC-Si Silica, Supelco). The extract solution was concentrated to dryness under a flow of N2 flow and the residue redissolved in 500 μL of acetonitrile. This sample solution was analyzed by HPLC. For nitro-PAH analysis, the residue was dissolved in 2 mL of DCM, and the solution was cleaned by SPE with alumina-acidic and alumina-basic solid phases (Bond Elut JR-AL-A and JR-AL-B, Varian). The extract solution was then concentrated to dryness under a flow of N2 followed by dissolution in 300 μL of ethanol/water (3/1, v/v). This sample solution was analyzed by HPLC.
PAHs were determined by HPLC with fluorescence detection. The system consisted of the detector (RF-10AXL, Shimadzu), a pump (LC-10AD, Shimadzu), a system controller (SCL-10A, Shimadzu), a degasser (DGU-20A5, Shimadzu), a column oven (CTO-10Avp, Shimadzu), and a column (3.0 mm i.d.×250 mm, Pegasil ODS, Senshu Pak). The mobile phase was acetonitrile/water (8/2, v/v). The flow rate was 0.5 mL min-1. The time program of the fluorescence detector was set to detect at the optimum excitation and emission wavelength for each target PAH.
For nitro-PAH analysis, the in-line reduction and chemiluminescence detection method using HPLC was employed, following a previously reported method (Murahashi and Hayakawa, 1997). The system consisted of a chemiluminescence detector (CLD-10A, Shimadzu), four pumps (LC-10ADvp and LC-10ATvp, Shimadzu), a six-port switching valve (FCV-12AH, Shimadzu), a column oven (CTO-10ACvp, Shimadzu), and a system controller (CBM-20A, Shimadzu). Two separation columns (3.0 mm i.d.×250 mm, 201TP54, Vydac and 5C18-MS-II, Nakalai Tesque), a concentration column (2.0 mm i.d.×5 mm, Cadenza CD-C18, Imtakt), and a reducing column (4.0 mm i.d.×10 mm, NPpak-R, Jasco) were employed. The mobile phase for first separation and reduction of nitro-PAHs was 75% ethanol-acetate buffer (pH 5.5), and that for the second separation was acetonitrile/imidazole-perchloric acid buffer (pH 7.6) (1 : 1, v/v). Each flow rate was 0.5 mL min-1. The mobile phase for concentration was 10 mM ascorbic acid in water, and the flow rate was 2 mL min-1 when necessary. The chemiluminescence reagent solution was 8 mM H2O2 and 0.64 mM bis (2,4,6-trichlorophenyl)oxalate in acetonitrile.
2. 4 Analysis of EC
EC was determined by the thermal oxidation method (Tanner et al., 1982) using CHN-CORDER (MT-3, Yanako). Carbonaceous components that were oxidized over a copper oxide catalyst under helium flow below 550°C were considered as organic carbon (OC). The remainders of the carbonaceous components, oxidized above 950°C in helium flow containing 10% oxygen, were assigned as EC.
3. RESULTS AND DISCUSSION
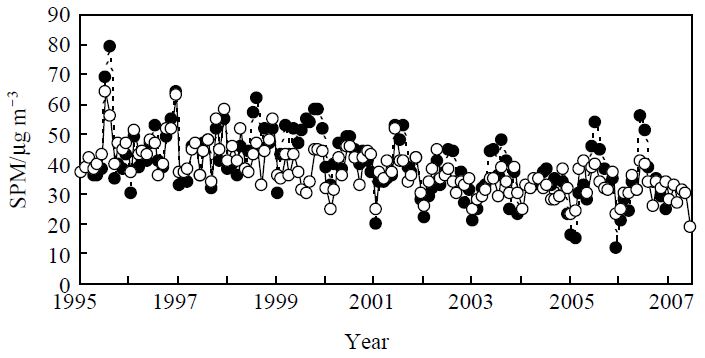
The variation in annual concentration of SPM at sites A and B, located in urban downtown Tokyo, were provided by government pollution-monitoring stations located close to each site, at Shirokane Station in Minato Ward (site A) and Honcho Station in Shinjuku Ward (site B). The SPM concentrations at the two sites were almost the same, and slightly decreased over the ten-year period studied (Fig. 2). The annual means of SPM concentration in 1997 and 2006 at site B were 44 μg m-3 and 33 μg m-3, respectively. This decrease suggests an improvement in the purification techniques for exhaust from combustion processes, such as in vehicles or power plants. Fig. 3 compares the annual mean concentrations of CO, NOx (NO+NO2), Oxidant (Ox), SPM, EC, PAHs, and nitro-PAHs from 1997-1998 (site A) to 2006-2007 (site B). Ox concentrations were based on the ultraviolet absorption method (APOA-370, Horiba). The SPM and gaseous concentrations were obtained by recalculating the data provided from the Honcho environmental monitoring station in Shinjuku Ward (site B). The P value is the statistical difference level calculated by a two-sided t-test. The SPM concentration slightly decreased from 34 μg m-3 in 1997-1998 to 29 μg m-3 in 2006-2007, in parallel with the results shown in Fig. 2. Similarly, the mean concentrations of NOx and Ox also slightly decreased. In contrast to our results, the annual mean concentration of Ox in Japan, including downtown Tokyo, has recently been reported to be constant or slightly increased in contrast to SPM and other gaseous pollutants such as CO and NOx, which are the major primary emissions from combustion (Tokyo Metropolitan Government, 2006; Ohara and Sakata, 2003). However, in our study the decrease was not statistically significant (P<0.05) and the P value was much higher than those for primary emissions such as CO, PAHs and 1-NP, this could be due to the Ox concentration covering a wide range of values in this study and could explain the discrepancy with the previous research.
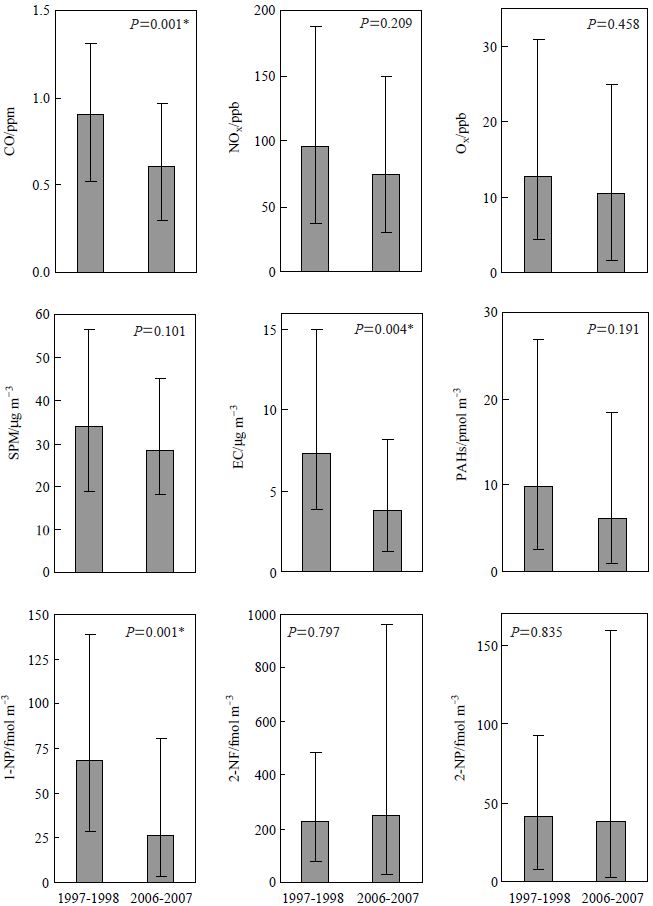
Comparison of annual mean concentrations of CO, NOx, Ox, SPM, EC, selected PAHs, and nitro-PAHs in 1997-1998 (site A) and in 2006-2007 (site B), and the statistical difference level (P, two-sided t-test). The PAH concentration is the sum of the concentrations of FL, PY, BkF, BaP, BghiP, IP, and PER. The column and error bar represent the annual mean concentration and the concentration range, respectively. Asterisks indicate a significant difference between the two sites (P<0.05).
Our results also showed that the annual mean concentrations of EC, primary PAHs and nitro-PAHs associated with airborne PM were much lower in 2006-2007 than in 1997-1998. The decrease in the atmospheric concentration of 1-nitropyrene (1-NP) and EC was particularly remarkable and statistically significant (P<0.01). A comparison of the annual-mean concentration ratios of SPM, PAHs, EC, and 1-NP in 2006-2007 to that in 1997-1998 showed they were in order SPM>PAHs>EC>1-NP(Table 1). The slight decrease of the mean SPM concentration compared with EC, PAHs, and 1-NP could be due to the variety of emission sources for airborne PM over and above primary combustion. Secondary aerosol formation from the oxidation of volatile organic compounds (VOCs) would also contribute significantly to changes in airborne PM concentration (Carreras-Sospedra et al., 2005; Eatough et al., 2003). The decrease in 1-NP concentration was the most significant, a possible explanation for this is the simultaneous reduction of both PM and NOx from diesel engines, where the higher-temperature combustion required for nitro-PAH formation from PAHs and NOx takes place (Tang et al., 2005). Over the time period of this study there were no large climate events, such as precipitation or wind. Even if the climate events influenced the atmospheric concentration of SPM, they did not influence the composition of primary emissions associated with airborne PM. Thus it was concluded that the regulation for DEP, which came into effect in Tokyo in 2003, led to a significant decrease of these compounds.

The annual mean atmospheric concentration and the ratios of SPM, PAHs, EC and 1-NP from 2006-2007 compared with those from 1997-1998.
In contrast to the decrease observed in direct emissions such as PAHs and 1-NP, the concentrations of nitro-PAHs, which are likely formed by atmospheric reaction of PAHs with NO2 (e.g 2-NF), remained the same or even slightly increased (Fig. 3). The concentration ratios for these secondary formed nitro-PAHs to 1-NP or BkF (nitro-PAH/1-NP or nitro-PAH/BkF ratio) also significantly increased (P<0.05) compared with those in 1997-1998, with the exception of 2-NP/BKF (Fig. 4). This indicated that atmospheric nitro-PAH formation was influenced by not only the concentration of the parent PAHs and NO2 but also other factors such as concentration of OH radicals, NO3 radicals, and other oxidants. Our results are consistent with a previous report that investigated diurnal changes of nitro-PAHs (Kameda et al., 2004a).
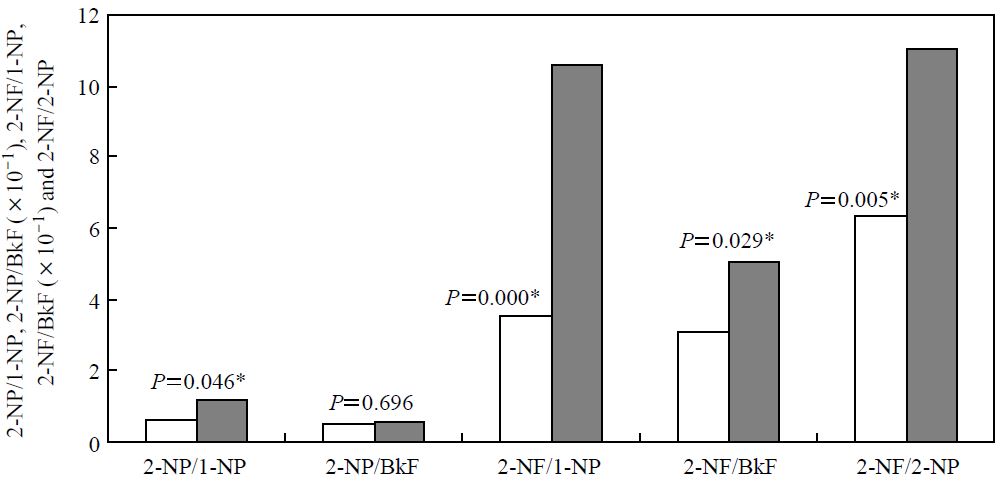
The concentration ratios of PACs observed in 1997-1998 (site A, white bar) and in 2006-2007 (site B, gray bar), and the statistical difference level (P, two-sided t-test). Asterisks indicate a significant difference between the two sites (P<0.05).
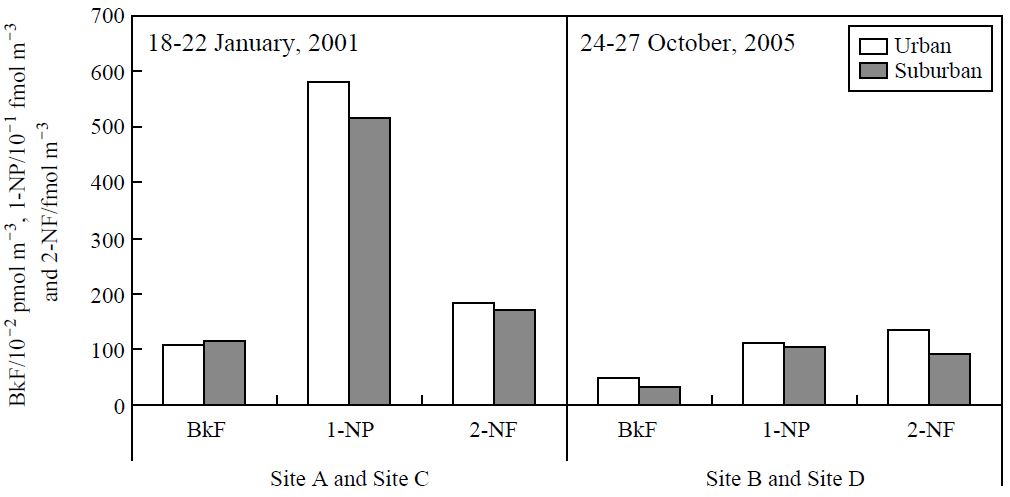
The average concentrations of BkF, 1-NP, and 2-NF in urban and suburban area observed in 18-22 January 2001 (sites A and C) and in 24-27 October 2005 (sites B and D).
In addition, the mean 2-NF/2-NP ratio significantly increased (P<0.01) (Fig. 4). 2-NF is known to be formed via both OH and NO3 radical initiated reactions of the parent PAH, FL (Arey et al., 1986). In comparison, 2-NP is formed only via OH radical-initiated reaction of PY (Zielinska et al., 1986). The relative importance of these two pathways for nitro-PAH formation can be determined from the 2-NF/2-NP ratio; several reports have utilized this index to discuss the occurrence of radical initiated-formation (Kameda et al., 2004a; Bamford and Baker, 2003; Feilberg et al., 2001). The increase in the 2-NF/2-NP ratio between 1997-1998 and 2006-2007 indicates that the NO3 radical-initiated nitration of PAHs has become more important. However, it has been reported that a 2-NF/2-NP ratio of 5-10 shows the OH-initiated reaction dominates when gas-phase concentrations of FL and PY are comparable, whereas the ratio will be more than 100 when the NO3-initiated reaction dominates (Bamford and Baker, 2003; Feilberg et al., 2001). In this study, the mean 2-NF/2-NP ratios in 1997-1998 and in 2006-2007 were 6.3 and 11.4, respectively. Thus, although the relative importance of the NO3 radical-initiated nitration of PAHs has increased, the OH radical-initiated reaction is still the major pathway for atmospheric formation of these nitro-PAHs.
As mentioned above, the Tokyo metropolitan government regulation on PM in diesel vehicle emissions resulted in changes, not only in the atmospheric concentration, but also in the composition of mutagenic PACs (indicated by 2-NF/BkF and 2-NF/1-NP ratios) associated with airborne PM in downtown Tokyo. Emission controls were also applied in neighboring prefectures such as Saitama, Chiba, and Kanagawa. Thus, the influence of the regulation was also examined at sites C and D (Fig. 1), which were located in suburban areas with lower population density than downtown Tokyo. The concentration levels of primary emissions from vehicles have been reported to be lower in suburban areas than in urban areas (Kameda et al., 2004b; Bamford and Baker, 2003; Hayakawa et al., 2002). However, no significant difference (P<0.05) in the mean concentrations of PACs was observed between the urban and suburban sites in either January 2001 (sites A and C) or October 2005 (sites B and D) (Fig. 4). Table 2 shows the mean 2-NF/BkF and 2-NF/1-NP ratios at four sampling sites in January and October. Even though the climate conditions in October are somewhat different to those in January, the mean 2-NF/1-NP and 2-NF/BkF ratios tended to increase after the implementation of control in 2003. These results indicate that emission control affected the chemical composition of airborne PM not only in urban Tokyo but also in the surrounding area.
4. CONCLUSIONS
This study revealed the effectiveness of the recently issued regulation on vehicle emissions as follows: 1. The government emission control, which began for vehicles in Tokyo in 2003, has worked effectively to remarkably decrease the atmospheric concentrations of primary emissions, especially those from dieselvehicles, such as 1-NP and EC; 2. The annual mean concentration of secondary-formed nitro-PAHs, 2-NF and 2-NP, has remained the same level suggesting other factor (s) than the concentration of parent PAHs and NO2 affect the degree of atmospheric formation of nitro-PAHs; 3. The 2-NF/2-NP ratio has clearly increased, indicating that the degree of atmospheric formation of nitro-PAHs by the NO3 radical-initiated reaction is elevated.
References
-
Arey, J., Zielinska, B., Atkinson, R., Winer, A.M., Ramdahl, T., Pitts, J.N. Jr., (1986), The formation of nitro-PAH from the gas-phase reactions of fluoranthene and pyrene with the OH radical in the presence of NOx, Atmospheric Environment, 20, p2339-2345.
[https://doi.org/10.1016/0004-6981(86)90064-8]

-
Bamford, H.A., Baker, J.E., (2003), Nitro-polycyclic aromatic hydrocarbon concentrations and sources in urban and suburban atmospheres of the mid-Atlantic region, Atmospheric Environment, 37, p2077-2091.
[https://doi.org/10.1016/s1352-2310(03)00102-x]

-
Carreras-Sospedra, M., Griffin, R.J., Dabdub, D., (2005), Calculations of incremental secondary organic aerosol reactivity, Environmental Science and Technology, 39, p1724-1730.
[https://doi.org/10.1021/es0495359]

-
Eatough, D.J., Long, R.W., Modey, W.K., Eatough, N.L., (2003), Semivolatile secondary organic aerosol in urban atmospheres: Meeting a measurement challenge, Atmospheric Environment, 37, p1277-1292.
[https://doi.org/10.1016/s1352-2310(02)01020-8]

-
Enya, T., Suzuki, H., Watanabe, T., Hirayama, T., Hisamatsu, Y., (1997), 3-Nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhaust and airborne particulates, Environmental Science and Technology, 31, p2772-2776.
[https://doi.org/10.1021/es961067i]

-
Feilberg, A., Poulsen, M.W.B., Nielsen, T., Skov, H., (2001), Occurrence and sources of particulate nitropolycyclic aromatic hydrocarbons in ambient air in Denmark, Atmospheric Environment, 35, p353-366.
[https://doi.org/10.1016/s1352-2310(00)00142-4]

-
Hayakawa, K., Tang, N., Akutsu, K., Murahashi, T., Kakimoto, H., Kizu, R., Toriba, A., (2002), Comparison of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in airborne particulates collected in downtown and suburban Kanazawa, Japan, Atmospheric Environment, 36, p5535-5541.
[https://doi.org/10.1016/s1352-2310(02)00252-2]

-
Hayakawa, K., Murahashi, T., Butoh, M., Miyazaki, M., (1995), Determination of 1,3-, 1,6-, and 1,8-dinitropyrenes and 1-nitropyrene in urban air by high-performance liquid chromatography using chemiluminescence detection, Environmental Science and Technology, 29, p928-932.
[https://doi.org/10.1021/es00004a012]

- Ishi, S., Hisamatsu, Y., Inazu, K., Aika, K., (2001), Environmental occurrence of nitrotriphenylene observed in airborne particulate matter, Chemosphere, 44, p681-690.
-
Kakimoto, H., Matsumoto, Y., Sakai, S., Kanoh, F., Arashidani, K., Tang, N., Akutsu, K., Nakajima, A., Awata, Y., Toriba, A., Kizu, R., Hayakawa, K., (2002), Comparison of atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in an industrialized city (Kitakyushu) and two commercial cities (Sapporo and Tokyo), Journal of Health Science, 48, p370-375.
[https://doi.org/10.1248/jhs.48.370]

- Kameda, T., Inazu, K., Bandow, H., Sanukida, S., Maeda, Y., (2004a), Diurnal change of direct-acting mutagenicity of soluble organic fraction of airborne particles collected at southern Osaka: Correlation between the mutagenicity, particle-associated nitroarenes, and gaseous emission, Atmospheric Environment, 38, p1903-1912.
- Kameda, T., Takenaka, N., Bandow, H., Inazu, K., Hisamatsu, Y., (2004b), Determination of atmospheric nitropolycyclic aromatic hydrocarbons and their precursors at heavy traffic roadside and at a residential area in Osaka, Japan, Polycyclic Aromatic Compounds, 24, p657-666.
-
Mastral, A.M., Callen, M.S., (2000), A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation, Environmental Science and Technology, 34, p3051-3057.
[https://doi.org/10.1021/es001028d]

-
Murahashi, T., Hayakawa, K., (1997), A sensitive method for the determination of 6-nitrochrysene, 2-nitrofuoranthene and 1-, 2-and 4-nitropyrenes in airborne particulates using high-performance liquid chromatography with chemiluminescence detection, Analytica Chimica Acta, 343, p251-257.
[https://doi.org/10.1016/s0003-2670(96)00632-0]

- Murahashi, T., Miyazaki, M., Kakizawa, R., Yamagishi, Y., Kitamura, M., Hayakawa, K., (1995), Diurnal concentrations of 1,3-,1, 6-,1,8-dinitropyrenes, 1-nitropyrene and benzo[a]pyrene in air in downtown Kanazawa and the contribution of diesel-engine vehicles, Japanese Journal of Toxicology and Environmental Health, 41, p328-333.
-
Nisbet, I.C.T., LaGoy, P.K., (1992), Toxic Equivalency Factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs), Regulatory Toxicology and Pharmacology, 16, p290-300.
[https://doi.org/10.1016/0273-2300(92)90009-x]

- Ohara, T., Sakata, T., (2003), Long-term observation of photochemical oxidants over Japan, Journal of Japan Society for Atmospheric Environment, 38, p47-54.
-
Phousongphouang, P.T., Arey, J., (2003), Soures of the atmospheric contaminants, 2-nitrobenzanthrone and 3-nitrobenzanthrone, Atmospheric Environment, 37, p3189-3199.
[https://doi.org/10.1016/s1352-2310(03)00344-3]

-
Pitts, J.N. Jr., Cauwenberghe, K.A.V., Schmid, J.P., Belser, W.L., Knudson, G.P., Hynds, P.M., (1978), Atmospheric reactions of polycyclic aromatic hydrocarbons: Facile formation of mutagenic nitro derivatives, Science, 202, p515-519.
[https://doi.org/10.1126/science.705341]

- Rosenkranz, H.S., Mermelstein, R., (1983), Mutagenicity and genotoxicity of nitroarenes. All nitro-containing chemicals were not created equal, Mutation Research, 114, p217-267.
-
Schuetzle, D., (1983), Sampling of vehicle emissions for chemical analysis and biological testing, Environmental Health Perspectives, 47, p65-80.
[https://doi.org/10.2307/3429500]

-
Tang, N., Hattori, T., Taga, R., Igarashi, K., Yang, X., Tamura, K., Kakimoto, H., Mishukov, V.F., Toriba, A., Kizu, R., Hayakawa, K., (2005), Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in urban air particulates and their relationship to emission sources in the Pan-Japan Sea Countries, Atmospheric Environment, 39, p5817-5826.
[https://doi.org/10.1016/j.atmosenv.2005.06.018]

-
Tanner, R.L., Gaffney, J.S., Phillips, M.F., (1982), Determination of organic and elemental carbon in atmospheric aerosol samples by thermal evolution, Analytical chemistry, 54, p1627-1630.
[https://doi.org/10.1021/ac00246a036]

- Tokyo Metropolitan Government Environmental White Paper 2006, http://www2.kankyo.metro.tokyo.jp/kouhou/env/eng/index.html.
- Yokota, H., (2007), Study on methods to reduce exhaust gases from heavy-duty diesel vehicles in use, Journal of Japan Society for Atmospheric Environment, 42, p1-15.
-
Zielinska, B., Arey, J., Atkinson, R., Ramdahl, T., Winer, A.M., Pitts, J.N. Jr., (1986), Reaction of dinitrogen pentoxide with fluoranthene, Journal of the American Chemical Society, 108, p4126-4132.
[https://doi.org/10.1021/ja00274a045]