Toxicological Effects of Polycyclic Aromatic Hydrocarbon Quinones Contaminated in Diesel Exhaust Particles
Abstract
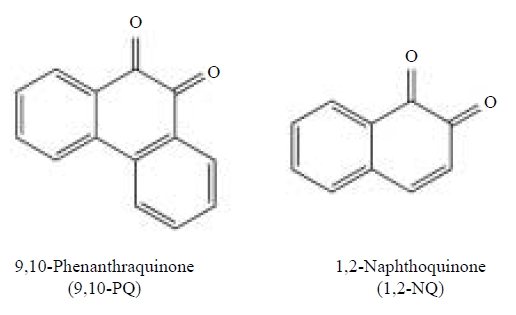
Accumulated epidemiological and animal studies have suggested that prolonged exposure to ambient particulate matter (PM) is associated with an increased risk of cardiovascular disease and pulmonary dysfunction. While diesel exhaust particles (DEP) contain large variety of compounds, polycyclic aromatic hydrocarbons (PAHs) are a dominant component contaminated in DEP. This article reviews effects of two PAH quinones, 9,10-phenanthraquinone (9,10-PQ) and 1,2-naphthoquinone (1,2-NQ), on vascular and respiratory systems.
Keywords:
Polycyclic aromatic hydrocarbon, Quinone, Oxidative stress, Vascular dysfunction, Diesel exhaust particle1. INTRODUCTION
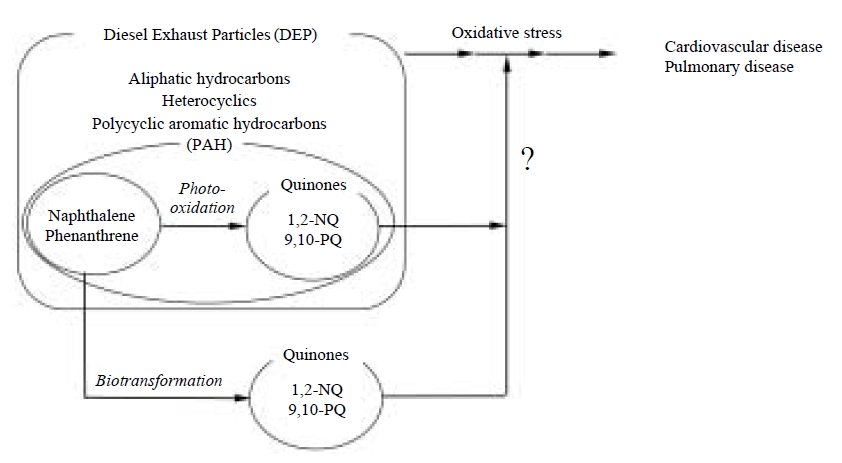
Diesel exhaust particles (DEP) are a major constituent of ambient particulate matter. A variety of organic compounds, such as aliphatic hydrocarbons, polycyclic aromatic hydrocarbons (PAHs) and heterocyclics are found in DEP (Wise et al., 2000; Simoneit et al., 1991; Ramdahl, 1985; Schuetzle, 1983; Schuetzle et al., 1981; Yu and Hites, 1981). The small size of DEP makes them easily inhaled, raising health concerns of their effects on lung cancer, allergy, asthma etc. (Nel et al., 2001; McClellan, 1987). It is well recognized that oxidative stress caused by DEP chemicals plays a critical role in these adverse effects (Xiao et al., 2003; Gavett and Koren, 2001; Takizawa et al., 2000; Li et al., 2000; Hiura et al., 1999; Lim et al., 1998; Sagai et al., 1993). Consistent with this notion, it was recently reported that there were organic chemicals showing redox activity in the airborne particulate matter (Cho et al., 2005). For this reason, identification of the substances in DEP which are involved in oxidative stress through excess amount of reactive oxygen species (ROS) is of interest for elucidating oxidative stress-dependent DEP toxicity.
Sagai et al. (1993) reported previously that formation of lung edema following intratracheal DEP injection into mice was markedly suppressed by pretreatment with polyethylene glycol-modified SOD (Sagai et al., 1993). They (Nagashima et al., 1995) subsequently showed that the DEP exposure resulted in the formation, in the mouse lung of 8-hydroxydeoxyguanosine, which is produced by hydroxyl radical (Kasai and Nishimura, 1984). These findings suggested that ROS such as hydroxyl radical derived from superoxide could be enzymatically and continuously generated from DEP and therefore overwhelm defenses. In 1997, we proposed the possibility that DEP components with a quinoid structure, undergo one-electron reduction by pulmonary NADPH-cytochrome P450 reductase to yield semiquinone radical which, in turn, can generate hydroxyl radical through a metal-catalyzed Haber-Weiss reaction through the redox-based generation of superoxide (Kumagai et al., 1997). Therefore, it is likely that such an overproduction of ROS contributes to, at least in part, the lung edema formation and 8-hydroxydeoxyguanosine production observed in vivo after exposure to DEP as shown in Fig. 1.
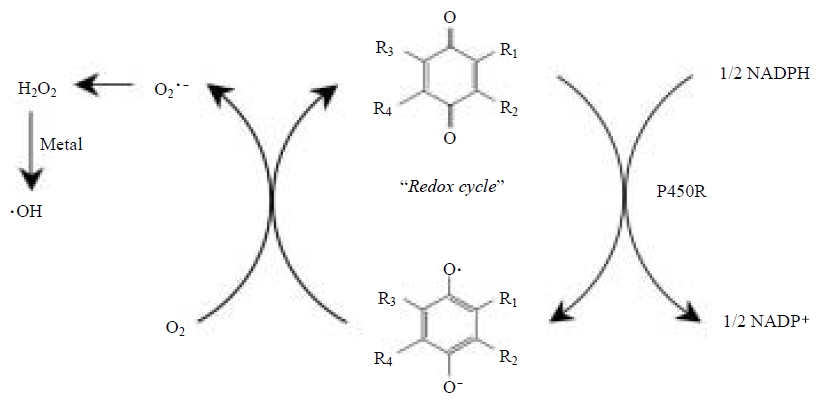
Metabolic activation of PAH quinones contaminated in DEP to generate reactive oxygen species. P450R, cytochrome P450 reductase.
PAH quinones with an α, β-unsaturated carbonyl group are electrophiles that react readily with nucleophiles such as protein thiolates to cause alkylation of crucial cellular proteins (Bolton et al., 2000; Monks et al., 1992; O’Brien, 1991). Alternatively, PAH quinones are highly redox active molecules that can be involved in the redox cycle with their semiquinone radical anions, resulting in the formation of ROS (Squadrito et al., 2001; Bolton et al., 2000; Penning et al., 1996). The current consensus is that PAH quinones are potential candidates for DEP-mediated oxidative stress (Baulig et al., 2003; Xiao et al., 2003; Nel et al., 2001; Li et al., 2000). DEP and diesel exhaust have been reported to contain a variety of PAH quinones such 9,10-phenanthraquinone (9,10-PQ), anthraquinone (AQ), naphthoquinone (NQ), naphthacenequinone, benzopyrenequinone, crysenequinone and fluorenequinone and possibly these derivatives (Cho et al., 2004; Leotz-Gartziandia et al., 2000; Allen et al., 1997; Schuetzle, 1983). We developed a quantitative method for determination of PQ, 9,10-AQ, 1,2-NQ and 1,4-NQ by electron impact gas chromatography-mass spectrometory (GC-MS) using selected ion monitoring after conversion of the quinones in the samples to their stable diacetyl derivatives (Cho et al., 2004). Using this procedure, the mean concentrations of 9,10-PQ, 9,10-AQ, 1,2-NQ, and 1,4-NQ were 24, 40, 14 and 8 μg per g of DEP (donated by Dr. H. Takano, National Institute for Environmental Studies, Japan), respectively. These results suggest that the levels of quinoid compounds in DEP are fairly low. However, our recent immunochemical study with specific antibody against 1,2-NQ revealed that naphthalene and its analogs, abundant combustion products in volatile-phase of the atmosphere (Fraser et al., 1998), are extensively biotransformed by enzyme systems in the body to form NQs (T. Miura et al., unpublished observation) and that the reactive quinones are covalently bound to macromolecules associated with irreversible toxicity (Zheng et al., 1997).
It should be, therefore, noted that the metabolic activation of PAHs (Smithgall et al., 1988) to quinones as well as photooxidation contributes to atmospheric quinone exposure (Squadrito et al., 2001) as shown in Fig. 2. In this article, the pathophysiological and toxicological actions of 9,10-PQ and 1,2-NQ (see Fig. 3) examined by our laboratory are reviewed.
2. DECREASED NITRIC OXIDE (NO) PRODUCTION AND MECHANISMS INVOLVED
Epidemiologic studies have suggested that exposure of humans to ambient particulate matter is associated with cardiopulmonary-related diseases and mortality (Pope et al., 2002, 1995; Dockery et al., 1993). NO is a biological signalling molecule that plays an important role in neurotransmission, vasorelaxation and immune response (Moncada et al., 1991) and is synthesized from L-arginine by NO synthase (NOS). All NOS isozymes consist of an N-terminal oxygenase domain and a C-terminal reductase domain, which is highly homologous with NADPH-cytochrome P450 reductase; this domain is capable of transferring electrons from NADPH to artificial acceptor molecules such as PAH quinones. Thus, we hypothesized that quinoid compounds could interact with the reductase domain on NOS, leading to a decrease in NO formation from L-arginine. Using rat cerebellar enzyme preparations that contained neuronal NOS, we showed that the inhibition of NO formation by quinones which exhibit a one-electron reduction potential (E1,7) ranging between -240 and -90 mV, increased at a more positive E1,7 (Kumagai et al., 1998b). Among 15 PAH quinones tested, 9,10-PQ (E1,7=-124 mV) and 1,2-NQ (E1,7=-89 mV), inhibited NO production most potently (IC50 value=10-12 μM) (Kumagai et al., 1998b). With purified neuronal NOS, we found that this enzyme effectively reduced the 9,10-PQ and 1,2-NQ, thereby causing a marked decrease in the production of NO from L-arginine (Kumagai et al., 1998b). In contrast, 9,10-AQ (E1,7=-348 mV) which showed negligible inhibitory effects on neuronal NOS activity, was not reduced by the enzyme. Taken together, we concluded that 9,10-PQ interacts with the P450 reductase domain on neuronal NOS, and inhibits NO formation by shunting electrons away from the normal catalytic pathway.
Ikeda et al. (1995) reported previously that incubation of rat aortic rings with suspensions of DEP resulted in a suppression of endothelium-dependent vasorelaxation caused by acetylcholine, suggesting that DEP components in urban air contribute to a disruption of vasorelaxation. Since impairment of NO production in the endothelium is implicated in the pathophysiology of vascular diseases (Kelly et al., 1996; Umans and Levi, 1995), we hypothesized that 9,10-PQ could inhibit the enzymatic activity not only of neuronal NOS but also of endothelial NOS, thereby altering NO formation, which could lead to suppression of endothelial NOS-dependent vasorelaxation and increased blood pressure. In a study using the total membrane fraction of bovine aortic endothelial cells, 9,10-PQ was found to be a more potent inhibitor of endothelial NOS than of neuronal NOS, with an IC50 value of 0.6 μM (Fig. 4A) (Kumagai et al., 2001). Endothelium-dependent relaxation of rat aortic rings by acetylcholine was significantly suppressed by 9,10-PQ (5 μM) (Fig. 4B). Moreover, exposure of rats to 9,10-PQ (0.36 mmol/kg) by intraperitoneal administration resulted in an elevation of blood pressure (1.4-fold). Under these conditions, systemic NO production, evaluated by plasma levels of NO metabolites in rats given 9,10-PQ, was reduced to 68% of control levels (Fig. 4C). These findings were consistent with the notion that 9,10-PQ and compounds like it, could be components of DEP that affect endothelial NOS activity, thereby suppressing NO-dependent vascular tone. To further substantiate this notion, we have recently reported that 1,2-NQ was a potent inhibitor of endothelial NOS as well (IC50 value=1.4 μM) and thus cause a profound suppression of acetylcholine-dependent vasorelaxation at a concentration of 5 μM(Sun et al., 2006).
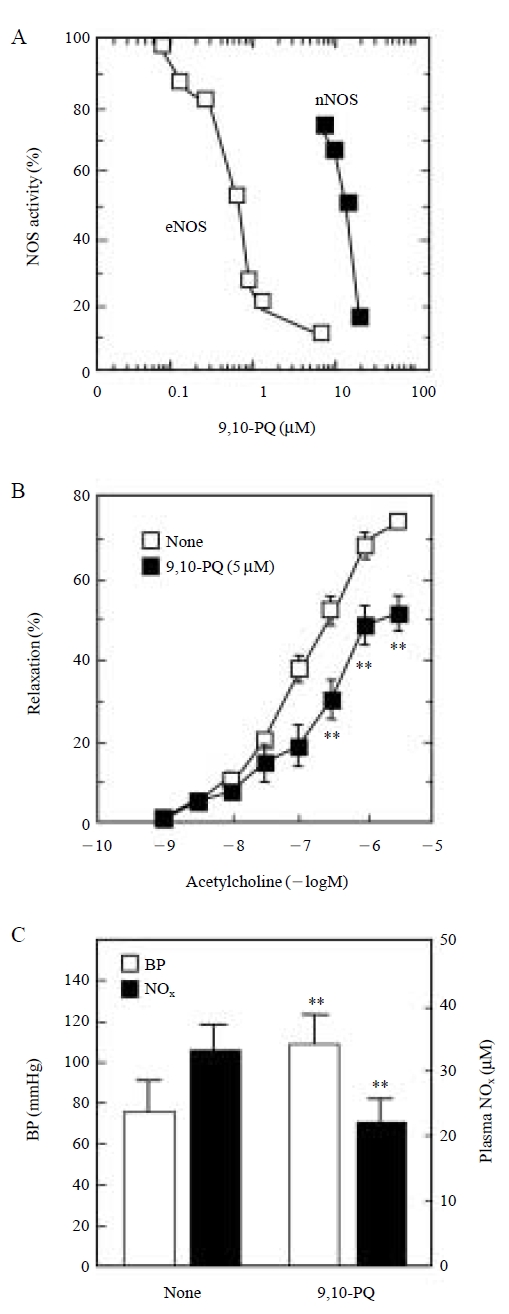
Changes in catalytic activities of NOS isoforms (A), endothelium-dependent relaxation of rat aorta (B), mean blood pressure in rats and plasma NOx levels in rats (C) by 9,10-PQ. 9,10-PQ, 9,10-phenanthraquinone; BP, blood pressure; NOx, nitric oxide metabolites (as an index for systemic NO production in vivo); nNOS, neuronal NOS; eNOS, endothelial NOS. **, P<0.01 vs. controls.
3. REDOX CYCLING OF 9,10-PQ AND OXIDATIVE STRESS
As mentioned earlier, quinones have two chemical characteristics, 1) covalent bond forming ability by electrophilic attack on nucleophilics, leading to thiol adduct formation and/or 2) redox cycling, in which their rapid reduction and oxidation results in generation of ROS (Bolton et al., 2000; Monks et al., 1992; O’Brien, 1991). Our studies indicated that 9,10-PQ reacts easily with dithiol compounds without loss of 9,10-PQ (Kumagai et al., 2002), indicating that the reactivity of 9,10-PQ toward dithiols is due to redox cycling. Such an interaction of dithiol with PAH quinones, resulting in thiol oxidation, was seen with 1,2-NQ, 1,4-NQ, 2,3-dichloro-1,4-NQ and juglone, whereas little appreciable oxidation was seen with 2-anilino-1,4-NQ, lapachol, 9,10-AQ and 5,12-naphthacenequinone. These results suggested that 9,10-PQ-mediated oxidation of proximal protein thiols and the reduction of molecular oxygen is involved in the destruction of cellular protein sulfhydryls.
Sugimoto et al. (2005) reported that exposure of human pulmonary epithelial A549 cells to 9,10-PQ induced apoptosis with a LC50 of ~7 μM. Oxidation of the cellular protein as determined by formation of protein carbonyls was also detected in cells after treatment with 9,10-PQ, suggesting that 9,10-PQ induces oxidative damage, presumably through generation of ROS. Interestingly, treatment of A549 cells with 10-20 μM 9,10-PQ for 12 h specifically down-regulated protein levels of Cu,Zn-superoxide dismutase and heme oxygenase-1 by more than 50% (Sugimoto et al., 2005). Thus, it seems likely that 9,10-PQ causes oxidative stress through not only redox cycling but also disruption of antioxidant defense system.
4. TRACHEAL CONTRACTION CAUSED BY 1,2-NQ AND MECHANISMS INVOLVED
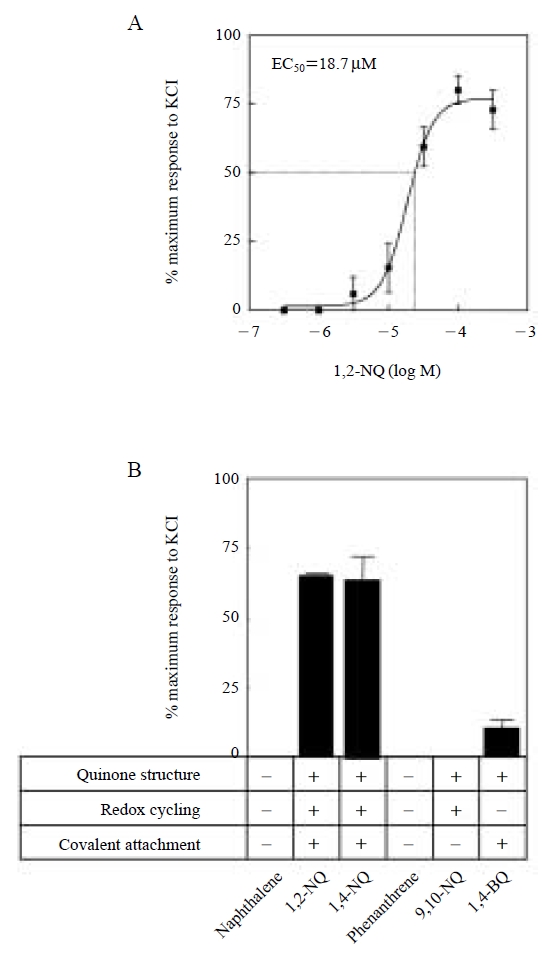
The exacerbation of asthma is one of the major adverse health effects of ambient particulate matter (PM). In studies of 1,2-NQ, we found that this quinone also mediates the contraction of tracheal smooth muscle but the effects were persistent. These persistent effects suggested that affected pulmonary tissue would become resistant to dilation, resulting in exacerbation of the asthma syndrome. Based on these considerations, we explored, ex vivo, the underlying mechanisms for this effect (Kikuno et al., 2006). We found that 1,2-NQ is capable of causing a concentration dependent contraction of tracheal smooth muscle in guinea pigs with EC50 value of 18.7 μM (Fig. 5A). At a concentration of 50 μM, the contraction intensities of 1,2-NQ, 1,4-NQ and 1,4-BQ were 75%, 63%, 11% of maximum response to 40 mM KCl, respectively, indicating a structural selectivity of the effect (Fig. 5B). The tricyclic quinones, 9,10-PQ, 9,10-AQ, and 5,12-naphthacenequinone, were without an effect on tracheal contraction even at 500 μM. Other polycyclic aromatic hydrocarbons such as naphthalene, anthracene, 1,2-benzanthracene, 2,3-benzofluorene, benzo[a]pyrene, chrysene, dibenzofuran, 3,6-dimetylphenanthrene, fluoranthrene, 2-nitropyrene, phenanthrene and pyrene did not affect tracheal tension under these conditions (Fig. 5B). These findings suggest that the tracheal contraction involves covalent attachment of the quinone group to protein cysteine residues (Zheng et al., 1997). Several lines of evidence suggested that 1,2-NQ activated phospholipase A2/lipoxygenase/vanilloid receptor signaling. More interestingly, 1,2-NQ was capable of transactivating protein tyrosine kinases (PTKs) such as epidermal growth factor receptor (EGFR) in guinea pig trachea, suggesting that phosphorylation of PTKs contributes to 1,2-NQ-induced tracheal contraction. In vitro study with A549 cells and cell-free system with purified protein tyrosine phosphatase 1B (PTP1B) have revealed that the transactivation of EGFR caused by 1,2-NQ exposure is, at least in part, attributable to inactivation of PTP1B through covalent binding of 1,2-NQ to PTP1B (N. Iwamoto et al., unpublished observation).
5. PULMONARY TOXICITY OF PAH QUINONES IN VIVO
Takano and his associates reported previously that DEP enhance airway inflammation in mice (Ichinose et al., 1998; Takano et al., 1998, 1997). It was also reported that DEP exposure is associated with allergic inflammation and increased immunoglobulin levels (Casillas et al., 1999; Nel et al., 1998; Diaz-Sanchez et al., 1994). However, it remained to be identified which component (s) from DEP are responsible for such an action. When 9,10-PQ (1 μg/body) was intratracheally injected into mice, macrophage levels in the bronchoalveolar lavage fluid (BALF) were not significantly different from those of control animals, but there was evidence for induction of neutrophils and eosinophils 24 hr after injection of 9,10-PQ. At 48 hr, the levels of neutrophils and eosinophils in 9,10-PQ-exposed group were approximately 10.6 times (P<0.05) and 71.9 times (P<0.05), respectively, of those in control groups (Hiyoshi et al., 2005a). Under the conditions, 9,10-PQ exposure caused a significant enhancement of pulmonary expression of interleukin-5 (IL-5, 5.6-fold) and eotaxin (1.9-fold), but not IL-1β, IL-2, IL-4, GM-CSF, IFN-γ, TNF-α, KC, MIP1-α or MCP-1 in the lungs (Hiyoshi et al., 2005a). These findings suggest that intratracheal exposure of mice to 9,10-PQ by single administration induces recruitment of inflammatory cells through the local expression of IL-5 and eotaxin. In the presence of ovalbumin (OVA), 9,10-PQ (2.1 ng/body) significantly increased the numbers of eosinophils and mononuclear cells in BALF as compared to OVA alone (Hiyoshi et al., 2005b). In contrast, the numbers of these cells around the airways were not significantly different between OVA challenge and OVA plus 9,10-PQ challenge in lung histology. 9,10-PQ exhibited adjuvant activity for the OVA-specific production of IgG1 and IgE (Hiyoshi et al., 2005b). These observations indicated that 9,10-PQ can enhance immunogloblin production and the infiltration of inflammatory cells into alveolar spaces that are related to OVA.
We also investigated the effects of 1,2-NQ on antigen-related airway inflammation, local expression of cytokine proteins, and antigen-specific IgGs production in mice (Inoue et al., 2007). Intratracheal administration of 1,2-NQ into mice aggravated antigen-related airway inflammation characterized by infiltration of eosinophils and lymphocytes around the airways and an increase in goblet cells in the bronchial epithelium in a dose-dependent manner. Combined exposure to 1,2-NQ and antigen enhanced the local expression of IL-4, IL-5, eotaxin, MCP-1, and KC as compared with exposure to antigen or 1,2-NQ alone. It was also found that 1,2-NQ exhibited adjuvant activity for the antigen-specific production of IgG1 and IgG2a. Overall, it is suggested that 9,10-PQ and 1,2-NQ are PAH quinones that enhance antigen-related airway inflammation in vivo.
References
-
Allen, J.O., N.M. Dookeran, K. Taghizadeh, A.L. Lafleur, K.A. Smith, and A.F. Sarofim, (1997), Measurement of oxygenated polycyclic aromatic hydrocarbons associated with a size-segregated urban aerosol, Environ. Sci. Technol., 31, p2064-2070.
[https://doi.org/10.1021/es960894g]

-
Baulig, A., M. Garlatti, V. Bonvallot, A. Marchand, R. Barouki, F. Marano, and A. Baeza-Squiban, (2003), Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells, Am. J. Physiol. Lung. Cell. Mol. Physiol., 285, pL671-679.
[https://doi.org/10.1152/ajplung.00419.2002]

-
Bolton, J.L., M.A. Trush, T.M. Penning, G. Dryhurst, and T.J. Monks, (2000), Role of quinones in toxicology, Chem. Res. Toxicol., 13, p135-160.
[https://doi.org/10.1021/tx9902082]

-
Casillas, A.M., T. Hiura, N. Li, and A.E. Nel, (1999), Enhancement of allergic inflammation by diesel exhaust particles: permissive role of reactive oxygen species, Ann. Allergy. Asthma. Immunol., 83, p624-629.
[https://doi.org/10.1016/s1081-1206(10)62884-0]

- Cho, A.K., D.A. Schmitz, Y. You, C.E. Rodriquez, E.D. Stefano, Y. Kumagai, A.H. Miguel, A. Eiguren, T. Kobayashi, E. Avol, and J.R. Froines, (2004), Determination of four quinones in diesel exhaust particles, SRM 1649a and atmospheric PM2.5, Aerosol Science & Technology, 38, p1-14.
-
Cho, A.K., C. Sioutas, A.H. Miguel, Y. Kumagai, D.A. Schmitz, M. Singh, A. Eiguren-Fernandez, and J.R. Froines, (2005), Redox activity of airborne particulate matter at different sites in the Los Angeles Basin, Environ. Res., 99, p40-47.
[https://doi.org/10.1016/j.envres.2005.01.003]

-
Diaz-Sanchez, D., AR. Dotson, H. Takenaka, and A. Saxon, (1994), Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms, J. Clin. Invest., 94, p1417-1425.
[https://doi.org/10.1172/jci117478]

- Dockery, D.W., C.A. Pope 3rd., X. Xu. J.D. Spengler, J.H. Ware, M.E. Fay, B.G. Ferris Jr., and F.E. Speizer, (1993), An association between air pollution and mortality in six U.S. cities, N. Engl. J. Med., 329, p1753-1759.
- Fraser, M.P., G.R. Cass, B.R.T. Simoneit, and R.A. Rasmussen, (1998), Air quality model evaluation data for organics. 5. C-6-C-22 nonpolar and semipolar aromatic compounds, Environ. Sci. & Technol., 32, p1760-1770.
-
Gavett, S.H., and H.S. Koren, (2001), The role of particulate matter in exacerbation of atopic asthma, Int. Arch. Allergy. Immunol., 124, p109-112.
[https://doi.org/10.1159/000053685]

- Hiura, T.S., M.P. Kaszubowski, N. Li, and A.E. Nel, (1999), Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages, J. Immunol., 163, p5582-5591.
- Hiyoshi, K., H. Takano, K. Inoue, T. Ichinose, R. Yanagisawa, S. Tomura, A.K. Cho, J.R. Froines, and Y. Kumagai, (2005a), Effects of a single intratracheal administration of phenanthraquinone on murine lung, J. Appl. Toxicol., 25, p47-51.
- Hiyoshi, K., H. Takano, K.I. Inoue, T. Ichinose, R. Yanagisawa, S. Tomura, and Y. Kumagai, (2005b), Effects of phenanthraquinone on allergic airway inflammation in mice, Clin. Exp. Allergy., 35, p1243-1248.
-
Ichinose, T., H. Takano, Y. Miyabara, and M. Sagai, (1998), Long-term exposure to diesel exhaust enhances antigen-induced eosinophilic inflammation and epithelial damage in the murine airway, Toxicol. Sci., 44, p70-79.
[https://doi.org/10.1006/toxs.1998.2459]

-
Ikeda, M., M. Suzuki, K. Watarai, M. Sagai, and T. Tomita, (1995), Impairment of endothelium-dependent relaxation by diesel exhaust particles in rat thoracic aorta, Jpn. J. Pharmacol., 68, p183-189.
[https://doi.org/10.1254/jjp.68.183]

- Inoue, K.I., H. Takano, K. Hiyoshi, T. Ichinose, K. Sadakane, R. Yanagisawa, S. Tomura, and Y. Kumagai, (2007), Naphthoquinone enhances antigen-related airway inflammation in mice, Eur Respir J., in press.
-
Kasai, H., and S. Nishimura, (1984), Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents, Nucleic. Acids. Res., 12, p2137-2145.
[https://doi.org/10.1093/nar/12.4.2137]

- Kelly, R.A., J.L. Balligand, and T.W. Smith, (1996), Nitric oxide and cardiac function, Circ. Res., p79. 363-380.
- Kikuno., S., K. Taguchi, N. Iwamoto, S. Yamano, A.K. Cho, J.R. Froines, and Y. Kumagai, (2006), 1,2-Naphthoquinone activates vanilloid receptor 1 through increased protein tyrosine phosphorylation, leading to contraction of guinea pig trachea, Toxicol. Appl. Pharmacol., 210, p47-54.
-
Kumagai, Y., T. Arimoto, M. Shinyashiki, N. Shimojo, Y. Nakai, T. Yoshikawa, and M. Sagai, (1997), Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPHcytochrome P450 reductase and involvement of the bioactivation in the DNA damage, Free Radic. Biol. Med., 22, p479-487.
[https://doi.org/10.1016/s0891-5849(96)00341-3]

-
Kumagai, Y., T. Hayashi, T. Miyauchi, A. Endo, A. Iguchi, M. Kiriya-Sakai, S. Sakai, K. Yuki, M. Kikushima, and N. Shimojo, (2001), Phenanthraquinone inhibits eNOS activity and suppresses vasorelaxation, Am. J. Physiol. Regul. Integr. Comp. Physiol., 281, pR25-30.
[https://doi.org/10.1152/ajpregu.2001.281.1.r25]

-
Kumagai, Y., S. Koide, K. Taguchi, A. Endo, Y. Nakai, T. Yoshikawa, and N. Shimojo, (2002), Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles, Chem. Res. Toxicol., 15, p483-489.
[https://doi.org/10.1021/tx0100993]

- Kumagai, Y., H. Nakajima, K. Midorikawa, S. Homma-Takeda, and N. Shimojo, (1998b), Inhibition of nitric oxide formation by neuronal nitric oxide synthase by quinones: nitric oxide synthase as a quinone reductase, Chem. Res. Toxicol., 11, p608-613.
-
Leotz-Gartziandia, E., V. Tatry, and P. Carlier, (2000), Sampling and Analysis of Polycyclic Aromatic Hydrocarbons (PAHs) and Oxygenated PAH in Diesel Exhaust and Ambient Air, Polycyclic Aromatic Compounds, 20, p245-258.
[https://doi.org/10.1080/10406630008034789]

-
Li, N., M.I. Venkatesan, A. Miguel, R. Kaplan, C. Gujuluva, J. Alam, and A. Nel, (2000), Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element, J. Immunol., 165, p3393-3401.
[https://doi.org/10.4049/jimmunol.165.6.3393]

-
Lim, H.B., T. Ichinose, Y. Miyabara, H. Takano, Y. Kumagai, N. Shimojyo, J.L. Devalia, and M. Sagai, (1998), Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice, Free Radic. Biol. Med., 25, p635-44.
[https://doi.org/10.1016/s0891-5849(98)00073-2]

-
McClellan, R.O., (1987), Health effects of exposure to diesel exhaust particles, Annu. Rev. Pharmacol. Toxicol., 27, p279-300.
[https://doi.org/10.1146/annurev.pharmtox.27.1.279]

- Moncada, S., R.M. Palmer, and E.A. Higgs, (1991), Nitric oxide: physiology, pathophysiology, and pharmacology, Pharmacol. Rev., 43, p109-142.
-
Monks, T.J., R.P. Hanzlik, G.M. Cohen, D. Ross, and D.G. Graham, (1992), Quinone chemistry and toxicity, Toxicol. Appl. Pharmacol., 112, p2-16.
[https://doi.org/10.1016/0041-008x(92)90273-u]

-
Nagashima, M., H. Kasai, J. Yokota, Y. Nagamachi, T. Ichinose, and M. Sagai, (1995), Formation of an oxidative DNA damage, 8-hydroxydeoxyguanosine, in mouse lung DNA after intratracheal instillation of diesel exhaust particles and effects of high dietary fat and beta-carotene on this process, Carcinogenesis, 16, p1441-1445.
[https://doi.org/10.1093/carcin/16.6.1441]

-
Nel, A.E., D. Diaz-Sanchez, and N. Li, (2001), The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress, Curr. Opin. Pulm. Med., 7, p20-26.
[https://doi.org/10.1097/00063198-200101000-00004]

-
Nel, A.E., D. Diaz-Sanchez, D. Ng, T. Hiura, and A. Saxon, (1998), Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system, J. Allergy. Clin. Immunol., 102, p539-554.
[https://doi.org/10.1016/s0091-6749(98)70269-6]

- O’Brien, P.J., (1991), Molecular mechanisms of quinone cytotoxicity, Chem. Biol. Interact., 80, p1-41.
-
Penning, T.M., S.T. Ohnishi, T. Ohnishi, and R.G. Harvey, (1996), Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase, Chem. Res. Toxicol., 9, p84-92.
[https://doi.org/10.1021/tx950055s]

- Pope, C.A. 3rd, R.T. Burnett, M.J. Thun, E.E. Calle, D. Krewski, K. Ito, and G.D. Thurston, (2002), Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution, Jama, 287, p1132-1141.
- Pope, CA. 3rd, M.J. Thun, M.M. Namboodiri, D.W. Dockery, J.S. Evans, F.E. Speizer, and C.W. Jr. Heath, (1995), Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults, Am. J. Respir. Crit. Care. Med., 151, p669-674.
-
Ramdahl, T., (1985), Characterization of Polar Compounds Such as Polycyclic Aromatic Ketones in Air-Pollution Including Wood Smoke, Environ. Int., 11, p197-203.
[https://doi.org/10.1016/0160-4120(85)90013-3]

-
Sagai, M., H. Saito, T. Ichinose, M. Kodama, and Y. Mori, (1993), Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse, Free Radic. Biol. Med., 14, p37-47.
[https://doi.org/10.1016/0891-5849(93)90507-q]

-
Schuetzle, D., (1983), Sampling of vehicle emissions for chemical analysis and biological testing, Environ Health Perspect, 47, p65-80.
[https://doi.org/10.2307/3429500]

-
Schuetzle, D., FS. Lee, and T.J. Prater, (1981), The identification of polynuclear aromatic hydrocarbon (PAH) derivatives in mutagenic fractions of diesel particulate extracts, Int. J. Environ. Anal. Chem., 9, p93-144.
[https://doi.org/10.1080/03067318108071903]

-
Simoneit, B.R.T., G.Y. Sheng, X.J. Chen, J.M. Fu, J. Zhang, and Y.P. Xu, (1991), Molecular Marker Study of Extractable Organic-Matter in Aerosols from Urban Areas of China, Atmos. Environ., A-25, p2111-2129.
[https://doi.org/10.1016/0960-1686(91)90088-o]

- Smithgall, T.E., R.G. Harvey, and T.M. Penning, (1988), Spectroscopic identification of ortho-quinones as the products of polycyclic aromatic trans-dihydrodiol oxidation catalyzed by dihydrodiol dehydrogenase. A potential route of proximate carcinogen metabolism, J. Biol. Chem., 263, p1814-20.
-
Squadrito, G.L., R. Cueto, B. Dellinger, and W.A. Pryor, (2001), Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter, Free Radic. Biol. Med., 31, p1132-8.
[https://doi.org/10.1016/s0891-5849(01)00703-1]

-
Sugimoto, R., Y. Kumagai, Y. Nakai, and T. Ishii, (2005), 9,10-phenanthraquinone in diesel exhaust particles downregulates Cu, Zn-SOD and HO-1 in human pulmonary epithelial cells: intracellular iron scavenger 1,10-phenanthroline affords protection against apoptosis, Free Rad. Biol. Med., 38, p388-395.
[https://doi.org/10.1016/j.freeradbiomed.2004.11.003]

-
Sun, Y., K. Taguchi, D. Sumi, S. Yamano, and Y. Kumagai, (2006), Inhibition of endothelial nitric oxide synthase activity and suppression of endotheliumdependent vasorelaxation by 1,2-naphthoquinone, a component of diesel exhaust particles, Arch. Toxicol., 80, p280-285.
[https://doi.org/10.1007/s00204-006-0127-8]

-
Takano, H., T. Ichinose, Y. Miyabara, T. Yoshikawa, and M. Sagai, (1998), Diesel exhaust particles enhance airway responsiveness following allergen exposure in mice, Immunopharmacol. Immunotoxicol., 20, p329-336.
[https://doi.org/10.3109/08923979809038548]

-
Takano, H., T. Yoshikawa, T. Ichinose, Y. Miyabara, K. Imaoka, and M. Sagai, (1997), Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice, Am. J. Respir. Crit. Care. Med., 156, p36-42.
[https://doi.org/10.1164/ajrccm.156.1.9610054]

-
Takizawa, H., T. Ohtoshi, S. Kawasaki, S. Abe, I. Sugawara, K. Nakahara, K. Matsushima, and S. Kudoh, (2000), Diesel exhaust particles activate human bronchial epithelial cells to express inflammatory mediators in the airways: a review, Respirology, 5, p197-203.
[https://doi.org/10.1046/j.1440-1843.2000.00245.x]

-
Umans, J.G., and R. Levi, (1995), Nitric oxide in the regulation of blood flow and arterial pressure, Annu. Rev. Physiol., 57, p771-90.
[https://doi.org/10.1146/annurev.physiol.57.1.771]

- Wise, S.A., B.A. Benner, M.J.L. De Alda, B.J. Porter, D.L. Poster, L.C. Sander, and M.M. Schantz, (2000), Recent Developments in NIST Standard Reference Materials for Polycyclic Aromatic Hydrocarbons in Environmental Matrices, Polycyclic Aromatic Compounds, 19, p297-313.
-
Xiao, G.G., M. Wang, N. Li, J.A. Loo, and A.E. Nel, (2003), Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line, J. Biol. Chem., 278, p50781-50790.
[https://doi.org/10.1074/jbc.m306423200]

-
Yu, M.L., and R.A. Hites, (1981), Identification of organic compounds on diesel engine soot, Anal. Chem., 53, p951-954.
[https://doi.org/10.1021/ac00230a005]

-
Zheng, J., M. Cho, A.D. Jones, and B.D. Hammock, (1997), Evidence of quinone metabolites of naphthalene covalently bound to sulfur nucleophiles of proteins of murine Clara cells after exposure to naphthalene, Chem. Res. Toxicol., 10, p1008-1014.
[https://doi.org/10.1021/tx970061j]