Dry Capture of Low-level CO2 from Public Indoor Spaces using Chemically Modified Carbonaceous Adsorbents - A Review
Copyright © 2021 by Asian Association for Atmospheric Environment
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Although CO2 is prominent as the most important greenhouse gas, responsible for 64% of anthropogenic global warming, it is also a viable indicator for indoor air quality (IAQ). Due to the incessant increase in the human population and residence time indoors, the need to control indoor CO2 levels has become exigent. To this effect, dryphase removal technology via adsorption with zeolites, activated carbons (AC) and activated carbon fibers (ACFs) had sufficed. Chemically modified AC and ACF surfaces through alkali impregnation have been used to improve their selectivity toward CO2 at room temperature. Here we appraise the various methods in the literature and carry out performance evaluation based on the physical and chemical modification induced by the chemical agents and experimental conditions. This study reviews the improved adsorption of low concentration (0.3%) via surface reformation of commercial carbon-based adsorbents, and the highest adsorption capacity was 2.2 mmol/g CO2 at the indoor level, which was achieved by AC pellets doped with ammine functionalities.
Keywords:
Indoor Air Quality, CO2 adsorption, Activated carbon, Activated carbon fiber, Surface Chemistry1. INTRODUCTION
Aside from being the main anthropogenic contributor to global warming, CO2, an index indicating indoor air quality (IAQ), is harmful at relatively high concentrations, especially in confined spaces such as offices, schools, subway stations, cars, airplanes, and submarines. Indoor CO2 concentration (IAQCO2) above 1000 ppm, causes unpleasantness, fatigue and headache. Therefore, effective management of IAQCO2 is necessary for human health (Satish et al., 2012).
According to Park et al. (2020), IAQCO2 in classrooms usually falls in the range of 1,000 to 3,000 ppm. Elsewhere, office meeting rooms have exhibit between 446 and 1,450 ppm CO2 with normal ventilation, which could rise to 4,920 ppm without ventilation (Vehviläinen et al., 2016). When uncrowded, the IAQCO2 of subway cabins ranges from 550 to 2,680 ppm (Lee et al., 2014). However, it often exceeds 5,000 ppm during rush-hours. Due to the commonly elevated IAQCO2 in public indoor spaces, the need for its monitoring and control. As set by the Environmental Protection Agency (EPA), IAQCO2>1,000 ppm requires efficient control technologies to ensure the safety of humans during their regular long-duration operations in confined spaces (Vehviläinen et al., 2016).
Among the various techniques for CO2 capture, dryphase adsorption is the only process feasibly applicable to indoor scenarios. Considering the low-concentrations of IAQCO2, natural (inevitable) and artificial (necessary) sources, incessant generation, and evasiveness, an adsorbent with a high affinity for CO2 molecules, hydrophobicity, sanitary safety, ready availability, mechanical stability and easy regeneration is required for sustainable use. Such an adsorbent should be efficient enough to operate under ambient conditions.
Thus, this study comprehensively explores plausible techniques to capture CO2 at low levels of 3,000 ppm, as studied in some references (Jeong et al., 2019; Kim et al., 2017; Adelodun and Jo, 2013), focusing more on dry adsorption. In this review, ultra-high-efficiency materials such as metal organic frames (MOF), carbon nanotubes (CNT) and hierarchically porous zeolites including ZSM and SBA were not addressed due to their high cost and lack of feasibility for indoor fields.
2. ADSORPTION OF IAQCO2
In the past two decades, researchers have intensified efforts to develop adsorption technology to control of IAQCO2 at room temperature. Popular among the approaches is the use of amine-based or amine-functionalized adsorbents, derived by tethering amine groups onto supports by grafting or chemical impregnation and immobilization of liquid amines (Moloney et al., 2011).
When compared to other technologies, adsorption offers some distinct advantages, particularly for indoor air control through product recovery, non-necessity of pollutant control during product recycling, automation, selectivity at trace and ultra-trace levels, excellent control and response to process change, cost-effectiveness, environmentally friendliness, high adsorbent reusability cycles, high energy efficiency etc.
The major factors that influence adsorption include the chemical properties of the chemical properties of the adsorbate and adsorbent, the adsorbent’s surface area and porosity, experimental conditions such as temperature, humidity, flow rate, gas matrix, pressure, etc.
Solid adsorbents (in packed or fluidized beds) are more energy efficient than wet scrubbers, in which CO2 molecules dissolve into the fluid. Whereas in adsorption, the process involves either weak physisorption by van der Waals attractions or chemisorption by covalent bonding interactions between the CO2 molecules (adsorbate) and an adsorbent surface. However, no sharp distinction exists between physical and chemical adsorptions. Rather a somewhat gradual transition scaled by the strength of the interaction at the interface occurs. At the equilibrium point at which adsorption rate equals that of desorption, the CO2-laden solid can be refreshed by subjecting it to one of pressure, vacuum, or temperature swing adsorptions, i.e. PSA, VSA and TSA respectively. Recently, combinations of two or more of these techniques have been adopted to achieve faster and more efficient regeneration.
Zeolites, composed of Al and Si oxides, are an easily-attainable, commercially-available adsorbent with a high adsorption affinity for various gases. Zeolites are microporous crystalline materials with adsorptive and ion exchange properties, often used to capture gaseous pollutants due to their characteristic high surface area and three-dimensional pore structures (Ye et al., 2012; Liu et al., 2011). The type of surface cations, polarity, surface area, and pore volume determine zeolites’ adsorption capacities (Plaza et al., 2007).
Amongst the noble functional zeolitic crystals, ZSM structures are excellent ones purposefully designed for CO2 capture. These zeolites tend to maximize electrostatic interactions between the surface and CO2 molecules. Besides, metal organic framework (MOF) is another porous solid material of immense porosity up to the region of 10,000 m2/g (Ghanbari et al., 2020). These three-dimensional adsorbents consist of secondary building units and connectors, whose length can be adjusted depending on the target molecule’s kinetic diameter. However, the cost of gaseous adsorption using these ‘future’ materials is still high, and it also requires handling expertise (Jiao et al., 2016; Wang et al., 2014).
On the other hand, carbon-based adsorbents such as activated carbon (AC) and activated carbon fiber (ACF) are incredibly porous materials with appreciably high surface area to unit weight ratio. These carbon-based adsorbents are currently the most popularly used due to their ruggedness, robustness, relatively low-cost, and reusability (Yu and Chuang, 2017). Capturing indoor air pollutants using carbonaceous adsorbents at normal temperatures is considered sustainable. Thus, recent advances on the surface modification of carbon-based adsorbents to enhance their selective and capacitive IAQCO2 adsorption are appraised in this study.
Adsorption selectivity is the measure of the preferential attraction of a material towards one or a few components in a mixture or matrix in which efficient contact is feasible between the target and the material (Adelodun et al., 2015).
Selectivity in a physical adsorption system may depend on differences in either equilibrium or kinetics, but the great majority of adsorption separation depends on equilibrium- based selectivity (Clarkson and Bustin, 2000). The selective adsorption of CO2 has become a tremendous research interest in recent years. Most of these studies on the selective separation of CO2 from N2 atmospheres include the use of metal-organic frameworks (Wu et al., 2014; Chen et al., 2013; Hou et al., 2013), synthetic zeolites (Sethia et al., 2014; Dangi et al., 2012), polymers (Nagarkar et al., 2011) and carbon-based adsor-bents (Chandra et al., 2012; Thote et al., 2010; Plaza et al., 2009a).
When properly modeled, the experimental investigation for the selective capture of IAQCO2 proved that the matrix of the inlet gases should comprise other common air constituents, be they natural such as Ar and moisture or anthropogenic such as VOCs and particulate matters. Thus, with CO2 being the major weak Lewis acid in indoor air, its selective adsorption is feasible by increasing the surface basicity of the adsorbent (Adelodun et al., 2015).
3. ACTIVATED CARBON (AC)
Due to the established demerit of zeolites with CO2 adsorption at high humidity, activated carbons are sufficient as suitable alternatives. ACs do not require any moisture removal, and they present a high adsorption capacity at ambient pressure. Moreover, they can easily regenerate for multiple reuses with no or insignificant drop in original efficiency (Plaza et al., 2009b). The surface chemistry of AC can strongly affect the adsorption of CO2 (Pevida et al., 2008). Due to the acidic nature of CO2 with weak Lewis acid, it is expected that the introduction of Lewis bases onto the AC surfaces may increase the adsorbent’s affinity toward CO2 molecules. Besides, surface impregnation, basifying the AC surfaces also includes increasing the π-electron density of the basal planes, which has also been reported to exhibit basic properties (Lahaye, 1998). To achieve surface basification of AC, heat treatment and amine-doping such as amination and ammoxidation are the respective physical and chemical approaches popularly used (Dali et al., 2012). In addition to this, the CO2 capture capacity could be enhanced by engineering the porous structure of AC in a hierarchical design adapted for the adsorbate. Sevilla and Fuertes (2011) and Sevilla et al. (2011) reported that ACs predominated with 1-2 nm width pores often exhibit excellent CO2 adsorption selectivity, unlike those with narrower micropores or with a mesoporous pore network.
Elsewhere, some efforts had been exemplified to use AC to support the highly successful CO2 absorbents by implying monoethanolamine (MEA) and glycine (Gly). This approach was conceived to improve the basic adsorbents’ portability, a property imperative for indoor use (Lee et al., 2013). Later, chemical modification by alkalimetal ion exchange also was carried out on Gly-based absorbents (Lim et al., 2016). However, such alkali impregnation significantly reduce the adsorbent’s specific surface area and microporosity. Such depreciation in the textural properties resulted in a decrease in pure CO2 sorption implying low CO2 storage capability. Contrarily, the 5 M supported MEA exhibited significantly enhanced low-level CO2 capture capacity from 0.016 to 1.064 mmol/g consistently.
The surface of the chemically modified AC adsorbents as observed by X-ray photoelectron spectroscopy (XPS) was tethered with amine, pyrrole and quaternary-N. In reducing potency, pyridine, amine, and pyrrolic-N have been identified as N-functionalities that contribute significantly to CO2 selective adsorption (Adelodun et al., 2015; Lim et al., 2015).
3. 1 Amination of AC
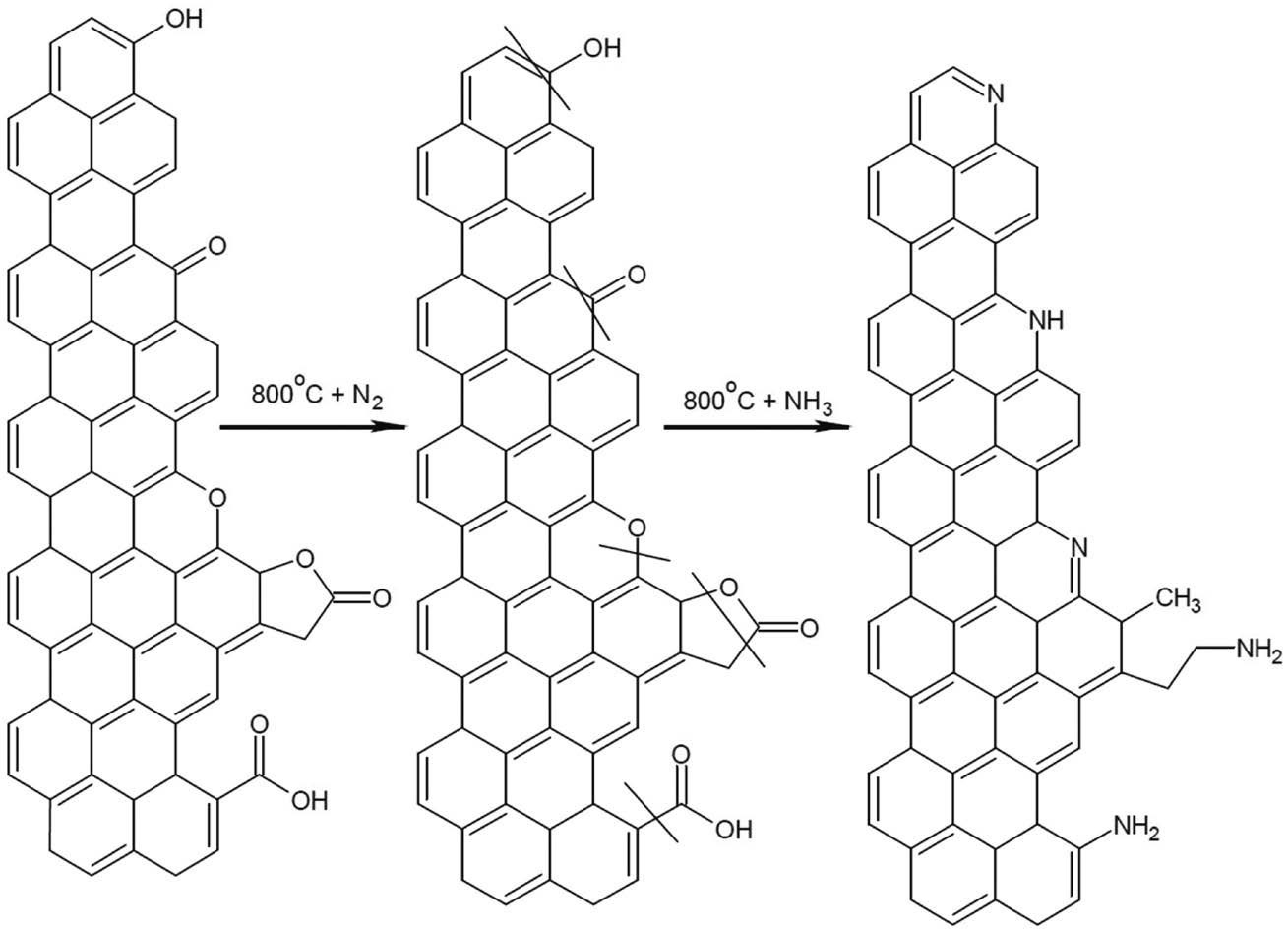
A highly bilateral interforce between adsorbent and CO2 molecules could be formed by providing strong covalent bonds with metal oxides as those found in MOFs. However, the financial cost to fabricate, handle, and regenerate MOFs are excessively high (DeSantis et al., 2017). For this reason, efforts have been made to up the selective efficiency of AC, being a cheaper and readily available alternative. In accordance, amine groups are on the AC surface using amination, a process in which thermally fragmentized ammonia radicals are impregnated on AC surfaces. The process is theoretically depicted in Fig. 1.
At temperature between 200 and 1, 200°C, the amine radicals viz. .NH2, :NH, atomic :N and .H, would attack the carbon surface to form surface nitrogen functionalities (SNFs) (Shafeeyan et al., 2010; Przepiorski et al., 2004; Soto-Garrido et al., 2003). Amination simply basifies the AC surface, turning it into a Lewis base-laden environment onto which CO2, a Lewis acid, could be easily scavenged. The pioneering studies on amination in the past decade are summarized in Table 1. Here, we found the stock material for the AC to vary widely.
3. 2 Ammoxidation of AC
Instead of pure ammonia gas used in amination, ammoxidation involves using ammonia-air gas mixture for nitrogen enrichment of carbon surface. Jansen and Bekkum (1994) observed differences in the surface chemistry of aminated and ammoxidized carbons carried out at 600- 900°C. Therein, they reported differences in the amidelactam- imide ratio between the techniques. The respective nitrogen contents and surface fractions obtained were 4.4 wt% and 0.14 wt% respectively for amination, while ammoxidation achieved 3.2 wt% and 0.11 wt%.
According to Pietrzak et al. (2007), ammoxidation with ammonia: air of 1 : 3 at 300°C, depends mainly on the period of the process ammoxidation is performed. They reported that the ammoxidation of brown coal decreases its surface area. Whereas, when used as a thermal activation process, it favors the formation of surface oxygen groups (SOFs) as well as enhancing the carbon’s basicity. However, using the similar ammoxidation condition (NH3 : air=1 : 3) but at 350°C, a further study carried out by Pietrzak (2009) on volatile bituminous coal resulted in enhancement of both the textural (pore structure and surface area) and surface basic property as varieties and large amount of SNFs were incorporated.
The thermogravimetric (TG-DTG) curves assessed by Wachowski and Hofman (2006) showed that coal samples ammoxidized at higher temperature exhibit slightly lower thermal stability. This occurrence could be due to the formation of less refractory SOFs usually associated with air-treatment at low temperatures.
A group of researchers investigated and identified a peculiar application of ammoxidized carbons. They reported their series of experimentals over five years, that ammoxidation could improve the capacitance of ACs. Their research focused on the capacitance behavior of ammoxidized coal and nanotube carbon materials purportedly designed for the electrical industry (Jurewicz et al., 2006, 2004 and 2002).
Shafeeyan et al. (2010) has provided the precise description SNFs plausibly found on activated carbons, especially after amination as depicted in Fig. 2. The research team saw the need to distinguish pyridine and pyridines from each other, mainly when the study of CO2 adsorption is concerned due to the varied affinity for the gas molecules.
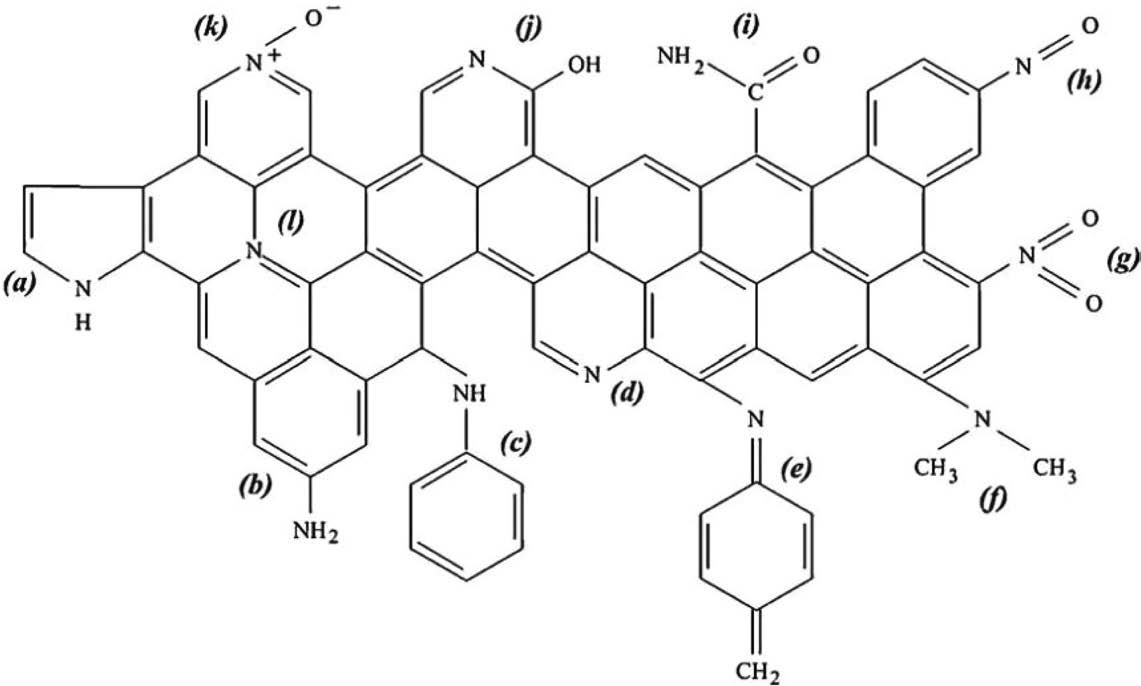
Detailed expression of nitrogen-containing complexes possibly found on activated carbons (Shefeeyan et al., 2010).
Chemical activation of carbon-based materials with a strong alkali such as concentrated KOH solution has been investigated and reported in recent works (Labus et al., 2014; Mitome et al., 2013). Identical methods that involve soaking and mixing the solid adsorbent with KOH solution, then sintering at a very high temperature under an inert atmosphere were used in all the reports. Usually, due to the increased instability of the carbon surface and the possible presence of highly reactive K2O, the treated carbons are then transferred into a concentrated acid solution (usually HCl) for neutralization. Some possible inside reactions are presented in equations (1) to (5). Results showed that improved microporosity achieved does enhance CO2 capacity capture of prepared adsorbents.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Before 2014, the reported CO2 capture selectivity at indoor levels by modified adsorbents did not show impressive achievement despite of several efforts for the adsorption of IAQ CO2 by AC. Even pre-oxidation treatment was later incorporated to populate the AC surface before the displacement reaction induced by the amine radicals. However, not until Adelodun et al. (2014b) adop- ted the alkalinization method was the target selectivity met. Later, the KOH treatment concept was adopted for basifying AC surface through alkylation, in which the thermally doped KOH was left on the AC by amine-stabilization (Adelodun et al., 2014b). It was the first time that sintered KOH could retain on a carbonaceous surface under air atmosphere without any burn-off due to oxidation. In their report, Adelodun et al. (2014b) were able to match the maximum adsorption capacity of modified AC (qmax (100%-CO2)) with its indoor (0.3%-CO2) selective adsorption capacity of 2.23 and 2.20 mmol/g, respectively.
4. ACTIVATED CARBON FIBER (ACF)
Generally activated carbon fibers (ACFs) used for CO2 adsorption are prepared from polymeric materials such as polyacrylonitrile (PAN). The systematic processes involved oxidation, activation and amination, focusing on the impregnating SNFs (Adelodun and Jo, 2013). Similar to the basification of AC, the specific surface area and pore volume of ACF were reduced after chemical amination. This is attributed to the presence of more SNFs on the external surface and inner pore walls. Amination is the substitution of a hydrogen bound to a carbon with an amino group.
In our previous studies, the ratio of micropores to the total volume ranged between 0.85 and 0.91, with an average pore width of 1.6 nm. Additionally, the induced SNFs such as imine, pyridine, and pyrrole present favorable affinity with CO2. The aminated ACF exhibited enhanced low-level (3,000 ppm) CO2 adsorption from 0.40 mmol/g at room temperature. The selective separation of CO2 against dry air (O2 & N2) also increased from 1.00 to 4.66 by amination.
4. 1 Alkalization of ACF
While amination is a dry-phase process where thermally decomposed ammonia gas impregnate basic Ngroups on the carbon surface, in this study, alkalization is the wet-phase alternative of concentrated solutions of alkali to incorporate alkali or alkali earth metals on the adsorbent. To optimize the advantage of fibrous adsorbents, activated carbon nanofibers (ACNFs) were fabricated via electrospinning, using polyacylonitrile (PAN) as a precursor (Kim et al., 2017). The spun fibers were 390 to 580 nm in thickness, with a surface area in the range of 27.3 to 300 m2/g. The surface structure was improved by programmed thermal activation at 800°C in a CO2 atmosphere, resulting in enhancement of specific surface area up to 542 m2/g. It was also found that the nitrogengroups including pyrrole and pyridine were created during the activation, which in turn facilitated the selective adsorption of CO2. The final adsorption capacity was 2.74 mmol/g for pure CO2 flow and 0.74 mmol/g for 3,000 ppm.
Further, the fabrication of doped-ACFs from melamine- blended polyacrylonitrile (MACNFs) improved their IAQ-CO2 removal (Jeong et al., 2019). Here, melamine was used as an alkali dopant for basic SNFs. Upon final CO2 activation, the specific surface area and microporosity were enhanced. As per chemical properties, the surface basicity was improved through the significant tethering of pyridine, allowing for preferential adsorption of CO2 over N2. The optimum melamine doping condition achieved the high CO2 adsorption capacity of 3.15 mmol/g.
Another attempt to tether SNFs on activated carbon nanofibers (ANF) was made using tetraethylenepentamine (TEPA) solution (Wang et al., 2020) Further impre- gnation of TEPA was achieved with preliminary oxidation of the nanofibers with 70% HNO3. The effects of HNO3 and TEPA treatments on the modified ANFs were found that although TEPA impregnation reduced the specific surface area and pore volume of the ANFs from 673.7 and 15.61 to 278.8 m2/g and 0.28 cm3/g, respectively, the HNO3 pre-oxidation increased the number of carboxylic groups on the ANF. Upon TEPA loading, pyridinic nitrogen was formed, which was further enhanced by pre-oxidation. The surface treatment cumulatively increased the amine content from 5.81% to 13.31%. The adsorbent’s final adsorption capacity toward 3,000 ppm and 100% CO2 levels improved from 0.20 and 1.89 to 0.33 and 2.96 mmol/g, respectively.
5. EVALUATION OF ADSORPTION CAPACITY
5. 1 Initial Adsorption Performance
Porous activated carbons are very effective for gas adsorption as driven by the sorbents’ pore structure and surface chemistry. Since the adsorbents are used for target harmful gases in the ambient condition, selectivity is an imperative property that ACs and ACFs should possess. For this reason, their capabilities for a lean gas treatment prioritized over their adsorption capacity for pure CO2, as required for CO2 storage and sequestration (Adelodun and Jo, 2013). The present review investigated mainly selective capture of CO2 in terms of chemical or physical modification of carbonaceous adsorbents.
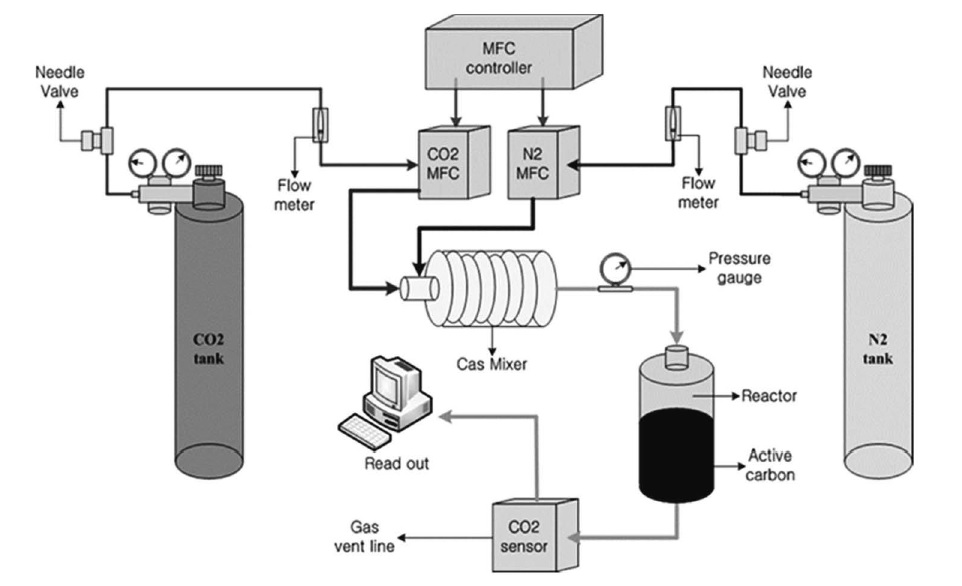
The adsorption capacity toward 3,000 ppm CO2 (in binary mixture with N2) was fed to the bed filled with adsorbents. Fig. 3 shows a typical lab-scale adsorption set-up for the evaluation (Adelodun et al., 2015; Lee and Jo, 2009). The SENSEair detector was equipped with a non-dispersive infra-red (NDIR) sensor for a continuous sampling. The amount of adsorbed CO2 (mmol/g) was evaluated using Equation (1):
| (1) |
where q=amount of CO2 adsorbed (mmol/g), Q= inflow rate of CO2 (200 mL/min), C0=CO2 inflow concentration (3000 ppm (i.e., 0.3%)), Ct=CO2 concentration (ppm) at saturation time, t=CO2 sensor intermittent read time (s), and M=mass of test sample (g).
As received ACs have been reported to exhibit 3.27 mmol/g adsorption capacity toward pure CO2 flow (Lim et al., 2013). However, its propensity for low level CO2 such as 0.3%, as frequently found in public indoor spaces, was extremely poor (0.016 mmol/g). To maintain a fresh and safe IAQ with less than 2000 ppm, various modifications on the carbonaceous adsorbents that have been experimented are summarized in Table 3. Focusing on field plausibility, commercial-grade alkaline reagents were used as dopants to improve the surface affinity of ACs and ACFs toward CO2.
Although, surface reformation often reduces the intrinsic specific surface area of carbonaceous adsorbents, causing a low adsorption tendency for pure CO2, such a modification adds bilateral interforce, resulting in high selectivity for low CO2 concentration.
Thus, the adsorption capacities of ACs and ACFs for pure CO2 are generally larger than 1 mmol/g, while those of IAQ-CO2 could be as high as 2.2 mmol/g.
Most surface treatments focus on textural modifications, increasing the proportion of supermicropores or ultramicropores with an appreciable degree of consistency. These micro scale pores are preferable for CO2 molecules whose kinetic diameter is approximately 3.3 nm.
5. 2 Regeneration and Reusability
One of the demerits of zeolites as a viable CO2 adsorbent is their affinity for moisture and high energy requirement for regeneration. The latter is due to the predominance of covalent bonding in attaching with CO2 during chemisorption (Lee et al., 2012). However, since carbon- based adsorbents interact with CO2 based on weaker physical or physical-chemical interactions, the energy requirement for their regeneration is lower, and hence more feasible (Jeon et al., 2016).
Usually, few adsorption studies attempt to perform regeneration studies after estimating the maximum adsorption capacity. And, because pure CO2 adsorption studies are more popular than those of low-level adsorptions, fewer the regeneration and reusability studies on the latter exist (Krishnamurthy et al., 2019). Notably, Adelodun et al. (2014b) achieved the highest adsorption amount for low-level (3,000 ppm) CO2 through fivecycle regenerations on the optimized sample. Generally, the regeneration of AC and ACF sorbents achieves adsorption capacity more than 90% of the initial cycle, provided the physical properties of the sorbent was not compromised during thermal or pressure swing adsorption refreshment.
6. MODELING OF ADSORPTION CHARACTERISTICS
During adsorption, the free, non-adsorbed gas and the adsorbed gas molecules are in a dynamic equilibrium, influenced by temperature, partial pressure or the adsorbate’s concentration, and the gas composition. The amount of adsorbate can be expressed using either surface concentration (mol/m2), or as surface coverage (θ, in %) which is defined as the ratio of the number of adsorption sites occupied to the number of adsorption sites available (Buekens et al., 2008).
The equilibrium partition between the free and the adsorbed state depends on temperature and pressure. It also can be described in terms of an empirical function .
The adsorption isotherm is the most widely used form for representing equilibrium data, because it is the most ameliorable with experimental data. Moreover, this is the form in which theoretical treatments are usually developed. Gas separation by adsorption is achieved by one of the three mechanisms viz steric, equilibrium or kinetic effect (Jasra et al., 1991). When the adsorption equilibrium state is determined at a constant temperature, an adsorption isotherm is obtained with changes in pressure. Adsorption isotherms can be used to estimate the surface area and pore volume in various porosity regimes, assessments of the surface chemistry of the adsorbent and fundamental information on the efficiency of various ACs.
The efficiency of adsorbents is estimated on the basis of its capacity, adsorption rate, mechanical strength, and possibility of regeneration and reusability. The adsorbent capacity is the most crucial parameter and the first often determined (Brdar et al., 2012).
Equilibrium data are fundamental information to design an adsorption process. At adsorption equilibrium, the chemical potential of the solute in the gaseous phase is equal to that in the solid phase. In this perspective, equilibrium relationships, describe how pollutants interact with adsorbents. Thus, they are critical for optimizing the adsorption pathways, expressing the surface properties and capacities of adsorbents, and the effective design of the adsorption system (El-Khaiary, 2008).
In general, an adsorption isotherm is an invaluable curve describing the phenomenon governing the retention (or release) or mobility of adsorbate from a fluidphase to a solid-phase at a constant temperature (Limousin et al., 2007). In our previous study (Adelodun et al., 2015), six models of the numerous isotherms available (Foo and Hamed, 2010) were employed to analyze the adsorption of gaseous CO2: 2-parameter models (Langmuir (L), Freundlich (F) and Temkin (Te) models) and 3-parameter ones (Sips (S), Toth (T) and Redlich-Peterson (R) models), as could be seen in Fig. 4.
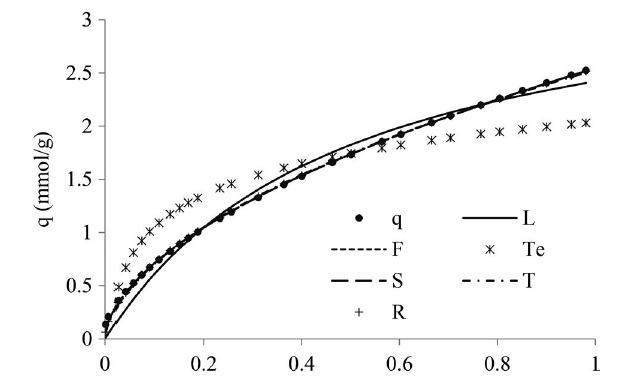
A typical isotherm model by non-linear fitting for pure CO2 flow with a surface aminated activated carbon.
Concerning the theoretical qmax values, only the Redlich-Peterson model was close to the experimental values. Meanwhile, Sips and Toth models, which have been reported in some other studies to exhibit a high degree of fitness with gas adsorption showed conformity to a reasonable extent (Adelodun et al., 2016). Freundlich is the most reliable two-parameter model to describe the adsorption of CO2 on either pristine or modified AC, while Sips and Redlich-Peterson models are better expressions, probably due to the extra parameter which improves their flexibility.
Therefore, the adsorption isotherm study confirms the presence of various SNGs (surface nitrogen groups) heterogeneously distributed on the modified AC. The process favors Freundlich and Langmuir isotherm at low or high CO2 concentrations, respectively (Lim et al., 2016).
7. CONCLUSIONS AND RECOMMENDATION
Despite the impressive adsorption capacities exhibited by activated carbons (ACs) and activated carbon fibers (ACFs), their selectivity toward indoor-level CO2 is poor. For this reason, chemical modification with various organic and inorganic dopants have been used to basify their surfaces, especially in the past two decades. Since the present review focuses on the field available process, high selectivity and capacity materials that are costly and imperfective molecular organic frames (MOFs) or novel mesoporous zeolitic structure were not considered.
We found that amination and ammoxidation offer varying degrees of success with improving both the surface chemistry and textural properties. In addition, some alkali materials such as glycine, urea, melamine and tetraethyleneamine etc. have been explored to modify the adsorbent surface including pore channels. In particular, the selective adsorption for low level CO2 capture could be improved by alkalinization with concentrated KOH.
Another carbonaceous adsorbent, ACF, is also useful in forming various functional groups suitable for CO2 molecules, because it can be designed to meet the specific purposes from the initial spinning step. The isotherm studies for this diluted CO2 capture seemed to result in Freundlich and Langmuir model implying simple gas adsorption.
The feasibility and performance evaluation for the real-life application of established CO2 adsorbents should be aimed from the view of practical environment. Based on the few empirical studies currently available on the CO2 adsorption from indoor spaces, the use of readily available materials such as waste, biomass, etc. for low-cost adsorbent preparation should be propagated to minimize regeneration cost. Process design studies including reactors further should continue with a focus on improving Indoor Air Quality (IAQ).
Acknowledgments
This study was supported by the National Research Foundation of Korea Grant funded by the Korea Government (MSIT, MOE) (No. 2019M3E7A1113077), and partially by Basic Research Fund (NRF-2015R1D1A1A01060182).
References
-
Adelodun, A.A., Jo, Y.-M. (2013) Integrated basic treatment of activated carbon for enhanced CO2 selectivity. Applied Surface Science, 286, 306-313.
[https://doi.org/10.1016/j.apsusc.2013.09.076]

-
Adelodun, A.A., Lim, Y.-H., Jo, Y.-M. (2014a) Effect of UV-C on pre-oxidation prior amination for preparation of a selective CO2 adsorbent. Journal of Analytical and Applied Pyrolysis, 105, 191-198.
[https://doi.org/10.1016/j.jaap.2013.11.004]

-
Adelodun, A.A., Lim, Y.-H., Jo, Y.-M. (2014b) Stabilization of KOH doping by amination on activated carbon for enhanced CO2 selective capture. Journal of Analytical and Applied Pyrolysis, 108, 151-159.
[https://doi.org/10.1016/j.jaap.2014.05.005]

-
Adelodun, A.A., Kim, K.-H., Ngila, J.C., Szulejko, J. (2015) A review on the effect on amination pretreatment for the selective separation of CO2. Applied Energy, 158, 631-642.
[https://doi.org/10.1016/j.apenergy.2015.08.107]

-
Adelodun, A.A., Ngila, J.C., Kim, D.G., Jo, Y.M. (2016) Isotherm, Thermodynamic and Kinetic Studies of Selective CO2 Adsorption on Chemically Modified Carbon Surfaces. Aerosol and Air Quality Research, 16, 3312-3329.
[https://doi.org/10.4209/aaqr.2016.01.0014]

-
An, H., Feng, B., Su, S. (2009) CO2 capture capacities of activated carbon fibre-phenolic resin composites. Carbon, 47(10), 2396-2405.
[https://doi.org/10.1016/j.carbon.2009.04.029]

-
Brdar, M., Šćiban, M., Takači, A., Došenović, T. (2012) Comparison of two and three parameters adsorption isotherm for Cr (VI) onto Kraft lignin. Chemical Engineering Journal, 183, 108-111.
[https://doi.org/10.1016/j.cej.2011.12.036]

- Buekens, A., Zyaykina, N.N., Xianwei, L. (2008) Pollution Control Technologies-Adsorption of Gaseous Pollutants, UNES CO-ELOSS.
-
Chandra, V., Yu, S.U., Kim, S.H., Yoon, Y.S., Kim, D.Y., Kwon, A.H., Meyyappan, M., Kim, K.S. (2012) Highly selective CO2 capture on N-doped carbon produced by chemical activation of polypyrrole functionalized graphene sheets. Chemical Communications, 48, 735-737.
[https://doi.org/10.1039/c1cc15599g]

-
Chen, D.S., Cheng, J.M., Sun, L.B., Liang, Z.Q., Shao, K.Z., Wang, C.G., Xing, H.-Z., Su, Z.-M. (2013) A new porous 2D copper (II) metal-organic framework for selective adsorption of CO2 over N2. Inorganic Chemistry Communications, 38, 104-107.
[https://doi.org/10.1016/j.inoche.2013.10.022]

-
Choma, J., Osuchowski, L., Marszewski, M., Dziura, A., Jaroniec, M. (2016) Developing microporosity in Kevlar®-derived carbon fibers by CO2 activation for CO2 adsorption. Journal of CO2 Utilization, 16, 17-22.
[https://doi.org/10.1016/j.jcou.2016.05.004]

-
Clarkson, C.R., Bustin, R.M. (2000) Binary gas adsorption/desorption isotherms: effect of moisture and coal composition upon carbon dioxide selectivity over methane. International Journal of Coal Geology, 42(4), 241-271.
[https://doi.org/10.1016/S0166-5162(99)00032-4]

- Dali, A.M., Ibrahem, A.S., Hadi, A. (2012) General study about activated carbon for adsorption of carbon dioxide. Journal of Purity, Utility Reaction and Environment, 1, 236-251.
- Dangi, G.P., Munusamy, K., Somani, R.S., Bajaj, H.C. (2012) Adsorption selectivity of CO2 over N2 by cation exchange zeolite L: Experimental and simulation studies. India Journal of Chemistry, 51A, 1238-1251.
-
DeSantis, D., Mason, J.A., James, B.D., Houchins, C., Long, J.R., Veenstra, M. (2017) Techno-economic analysis of metalorganic frameworks for hydrogen and natural gas storage. Energy Fuels, 31, 2024-2032.
[https://doi.org/10.1021/acs.energyfuels.6b02510]

-
Díez, N., Álvarez, P., Granda, M., Blanco, C., Santamaría, R., Menédez, R. (2015) CO2 adsorption capacity and kinetics in nitrogen-enriched activated carbon fibers prepared by different method. Chemical Engineering Journal, 281, 704-712.
[https://doi.org/10.1016/j.cej.2015.06.126]

-
El-Khaiary, M.I. (2008) Least-squares regression of adsorption equilibrium data: Comparing the options. Journal of Hazardous Materials, 158, 73-87.
[https://doi.org/10.1016/j.jhazmat.2008.01.052]

-
Foo, K.Y., Hameed, B.H. (2010) Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156(1), 2-10.
[https://doi.org/10.1016/j.cej.2009.09.013]

-
Ghanbari, T., Abnisa, F., Wan Daud, W.M.A. (2020) A review on production of metal organic frameworks (MOF) for CO2 adsorption. Science of The Total Environment, 707, 135090.
[https://doi.org/10.1016/j.scitotenv.2019.135090]

-
Gray, M.L., Soong, Y., Champagne, K.J., Baltrus, J., Stevens Jr, R.W., Toochinda, P., Chuang, S.S.C. (2004) CO2 capture by amine-enriched fly ash carbon sorbents. Separation and Purification Technology, 35(1), 31-36.
[https://doi.org/10.1016/S1383-5866(03)00113-8]

-
Hong, H.-E., Adeloudun, A.A., Jo, Y.-M. (2013) Preparation of KOH Impregnated AC Pellets for Selective CO2 Capture. Journal of Korean Society of Odor Research and Engineering, 12(4), 203-210.
[https://doi.org/10.11161/jkosore.2013.12.4.203]

-
Hou, C., Liu, Q., Wang, P., Sun, W.-Y. (2013) Porous metalorganic frameworks with high stability and selective sorption for CO2 over N2. Microporous and Mesoporous Materials, 172, 61-66.
[https://doi.org/10.1016/j.micromeso.2013.01.020]

-
Huang, P.-H., Cheng, H.-H., Lin, S.-H. (2015) Adsorption of Carbon Dioxide onto Activated Carbon Prepared from Coconut Shells. Journal of Chemistry, 2015, 1-10.
[https://doi.org/10.1155/2015/106590]

-
Hwang, S.-H., Kim, D.-W., Jung, D.-W., Jo, Y.-M. (2016) Impregnation of Nitrogen Functionalities on Activated Carbon Fiber Adsorbents for Low-level CO2 Capture. Journal of Korean Society for Atmospheric Environment, 32(2), 176-183.
[https://doi.org/10.5572/KOSAE.2016.32.2.176]

-
Jansen, R.J.J., van Bekkum, H. (1994) Amination and ammoxidation of activated carbons. Carbon, 32(8), 1507-1516.
[https://doi.org/10.1016/0008-6223(94)90146-5]

-
Jasra, R.V., Choudary, N.V., Bhat, S.G.T. (1991) Separation of gases by pressure swin. Separation Science and Technology, 26(7), 885-930.
[https://doi.org/10.1080/01496399108050504]

-
Jeon, D.-H., Bae, S.-T., Par, S.-J. (2016) Preparation and characterization of chemically activated carbon materials for CO2 capture. Carbon Letters, 17(1), 85-89.
[https://doi.org/10.5714/CL.2016.17.1.085]

-
Jeong, D., Wang, J., Adelodun, A.A., Kim, S., Jo, Y. (2019) Electrospun melamine-blended activated carbon nanofibers for enhanced control of indoor CO2. Journal of Applied Polymer Science, 136(28), 47747.
[https://doi.org/10.1002/app.47747]

-
Jiao, J., Cao, J., Xia, Y., Zhao, L. (2016) Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chemical Engineering Journal, 306, 9-16.
[https://doi.org/10.1016/j.cej.2016.07.041]

-
Jurewicz, K., Babeł, K., Ziółkowski, A., Wachowska, H., Kozłowski, M. (2002) Ammoxidation of brown coals for supercapacitors. Fuel Processing Technology, 77-78, 191-198.
[https://doi.org/10.1016/S0378-3820(02)00069-3]

-
Jurewicz, K., Babeł, K., Ziółkowski, A., Wachowska, H. (2004) Capacitance behavior of the ammoxidized coal. Journal of Physics and Chemistry of Solids, 65(2-3), 269-273.
[https://doi.org/10.1016/j.jpcs.2003.10.023]

-
Jurewicz, K., Babeł, K., Pietrzak, R., Delpeux, S., Wachowska, H. (2006) Capacitance properties of multi-walled carbon nanotubes modifies by activation and ammoxidation. Carbon, 44(12), 2368-2375.
[https://doi.org/10.1016/j.carbon.2006.05.044]

-
Kim, D.W., Jung, D.W., Adelodun, A.A., Jo, Y.M. (2017) Evaluation of CO2 adsorption capacity of electrospun carbon fibers with thermal and chemical activation. Journal of Applied Polymer Science, 134(47), 45534.
[https://doi.org/10.1002/app.45534]

-
Krishnamurphy, A., Salunkhe, B., Zore, A., Rownaghi, A., Schuman, T., Rezaei, F. (2019) Amine-based latex coatings for indoor air CO2 control in commercial buildings. Applied Materials and Interfaces, 11, 16594-16604.
[https://doi.org/10.1021/acsami.9b02934]

-
Labus, K., Gryslewicz, S., Machnikowski, J. (2014) Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity, Fuel, 118, 9-15.
[https://doi.org/10.1016/j.fuel.2013.10.042]

-
Lahaye, J. (1998) The chemistry of carbon surfaces. Fuel, 77(6), 543-547.
[https://doi.org/10.1016/S0016-2361(97)00099-9]

- Lee, K.M., Jo, Y.M. (2009) Ambient Adsorption of Low-level Carbon Dioxide by Metal Treated Activated Carbon. Journal of Korean Society for Atmospheric Environment, 25, 316-324.
-
Lee, K.-M., Lim, Y.-H., Park, C.-J., Jo, Y.-M. (2012) Adsorption of Low-Level CO2 Using Modified Zeolites and Activated Carbon. Industrial & Engineering Chemistry Research, 51, 1355-1363.
[https://doi.org/10.1021/ie2013532]

-
Lee, K.B., Kim, J.S., Bae, S.J., Kim, S.D. (2014) Research Study on Indoor Air Quality (IAQ) inside of the Subway Cabin in Seoul Metropolitan City. Journal of Korean Society for Atmospheric Environment, 30(2), 175-187.
[https://doi.org/10.5572/KOSAE.2014.30.2.175]

-
Lee, S.-Y., Park, S.-J. (2013) Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. Journal of Colloid and Interface Science, 389(1), 230-235.
[https://doi.org/10.1016/j.jcis.2012.09.018]

-
Lee, H.-M., Kang, H.-R., An, K.-H., Kim, H.-G., Kim, B.-J. (2013) Comparative studies of porous carbon nanofibers by various activation methods. Carbon Letters, 14(3), 180-185.
[https://doi.org/10.5714/CL.2013.14.3.180]

- Lim, Y.-H., Park, Y.K., Jo, Y.-M. (2012) Characterization of Glycine Metal Salts for CO2 Absorption. Applied Chemistry for Engineering, 23(3), 284-288.
-
Lim, Y.-H., Jo, Y.-M., Kim, S.-H. (2013) Adsorption of Carbon Dioxide using Pelletized AC with Amine impregnation. Journal of the Korean Applied Science and Technology, 30(1), 88-95.
[https://doi.org/10.12925/jkocs.2013.30.1.088]

-
Lim, C.-H., Holder, A.M., Hynes, J.T., Musgrave, C.B. (2015) Catalytic Reduction of CO2 by Renewable Organohydrides. Journal of Physical Chemistry Letters, 6(24), 5078-5092.
[https://doi.org/10.1021/acs.jpclett.5b01827]

-
Lim, Y.H., Adelodun, A.A., Kim, D.W., Jo, Y.M. (2016) Surface Impregnation of Glycine to Activated Carbon Adsorbents for Dry Capture of Carbon Dioxide. Asian Journal of Atmospheric Environment, 10-2, 99-113.
[https://doi.org/10.5572/ajae.2016.10.2.099]

-
Limousin, G., Gaudet, J.-P., Charlet, L., Szenknect, S., Barthès, V., Krimissa, M. (2007) Sorption isotherms: A review on physical bases, modeling and measurement. Applied Geochemistry, 22(2), 249-275.
[https://doi.org/10.1016/j.apgeochem.2006.09.010]

-
Liu, Y., Ye, Q., Shen, M., Shi, J., Chen, J., Pan, H., Shi, Y. (2011) Carbon Dioxide Capture by Functionalized Solid Amine Sorbents with Simulated Flue Gas Conditions. Environmental Science & Technology, 45, 5710-5716.
[https://doi.org/10.1021/es200619j]

-
Mitome, T., Uchida, Y., Egashira, Y., Hayashi, K. Nishiura, A., Nishiyama, N. (2013) Adsorption of indole on KOH-activated mesoporous carbon. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 424, 89-95.
[https://doi.org/10.1016/j.colsurfa.2013.02.022]

-
Moloney, P., Huffman, C., Gorelik, O., Nikolaev, P., Arepalli, S., Allada, R., Springer, M., Yowell, L. (2011) Advanced life supports for space exploration: Air revitalization using amine coated single wall carbon nanotubes. MRS Online Proceedings Library Archive, 851, 59-64.
[https://doi.org/10.1557/PROC-851-NN2.4]

-
Nagarkar, S.S., Chaudhari, A.K., Ghosh, S.K. (2011) Selective CO2 adsorption in a robust and water-soluble porous coordination polymer with new network topology. Inorganic Chemistry, 51, 572-576.
[https://doi.org/10.1021/ic202102m]

-
Park, J.-H., Lee, T.J., Park, M.J., Oh, H., Jo, Y.M. (2020) Effects of air cleaners and school characteristics on classroom concentrations of particulate matter in 34 elementary schools in Korea. Building and Environment, 167, 106437.
[https://doi.org/10.1016/j.buildenv.2019.106437]

-
Pevida, C., Plaza, M.G., Arias, B., Fermoso, J., Rubiera, F., Pis, J.J. (2008) Surface modification of activated carbon for CO2 capture. Applied Surface Science, 254(22), 7165-7172.
[https://doi.org/10.1016/j.apsusc.2008.05.239]

-
Pietrzak, R., Wachowska, H., Nowiski, P., Babel, K. (2007) Preparation of modified active carbon from brown coal by ammoxidation. Fuel Processing Technology, 88(4), 409-415.
[https://doi.org/10.1016/j.fuproc.2006.11.001]

-
Pietrzak, R. (2009) XPS study and physic-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel, 88(10), 1871-1877.
[https://doi.org/10.1016/j.fuel.2009.04.017]

-
Plaza, M.G., Pevida, C., Arenillas, A., Rubiera, F., Pis, J.J. (2007) CO2 capture by adsorption with nitrogen enriched carbons. Fuel, 86(14), 2204-2212.
[https://doi.org/10.1016/j.fuel.2007.06.001]

-
Plaza, M.G., Pevida, C., Arias, B., Casal, M.D., Martin, C.F., Fermoso, J., Rubiera, F., Pis, J.J. (2009a) Different approaches for the development of low-cost CO2 adsorbents. Journal of Environmental Engineering, 135(6), 426-432.
[https://doi.org/10.1061/(ASCE)EE.1943-7870.0000009]

-
Plaza, M.G., Pevida, C., Arias, B., Fermoso, C.F., Rubiera, F., Pis, J.J. (2009b) A comparison of two methods for producing CO2 captures adsorbents. Energy Procedia, 1(1), 1107-1113.
[https://doi.org/10.1016/j.egypro.2009.01.146]

-
Plaza, M.G., Pevida, C., Arias, B., Fermoso, J., Casal, M.D., Martin, C.F., Rubiera, F., Pis, J.J. (2009) Development of low-cost biomass-based adsorbents for postcombustion CO2 capture. Fuel, 88(12), 2442-2447.
[https://doi.org/10.1016/j.fuel.2009.02.025]

-
Przepiorski, J., Skrodewicz, M., Morawski, A.W. (2004) High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Applied Surface Science, 225(1-4), 235-242.
[https://doi.org/10.1016/j.apsusc.2003.10.006]

-
Satish, U., Mendell, M.J., Shekhar, K., Hotchi, T., Sullivan, D., Streufert, S., Fisk, W.J. (2012) Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environmental Health Perspectives, 120, 1671-1677.
[https://doi.org/10.1289/ehp.1104789]

-
Sethia, G., Patel, H.A., Pawar, R.R., Bajaj, H.C. (2014) Porous synthetic hectorites for selective adsorption of carbon dioxide over nitrogen, methane, carbon monoxide and oxygen. Applied Clay Science, 91-92, 63-69.
[https://doi.org/10.1016/j.clay.2014.01.019]

-
Sevilla, M., Fuertes, A.B. (2011) Sustainable porous carbons with a superior performance for CO2 capture. Energy Environment Science, 4, 1765-1771.
[https://doi.org/10.1039/c0ee00784f]

-
Sevilla, M., Valle-Vigon, P., Fuertes, A.B. (2011) N-doped polypyrrole- based porous carbons for CO2 capture. Advanced Functional Materials, 21(14), 2781-2787.
[https://doi.org/10.1002/adfm.201100291]

-
Shafeeyan, M.S., Daud, W.M.A.W., Houshmand, A., Shamiri, A. (2010) A review on surface modification of activated carbon adsorption. Journal of Analytical and Applied Pyrolysis, 89(2), 143-151.
[https://doi.org/10.1016/j.jaap.2010.07.006]

-
Soto-Garrido, G., Aguilar, C., Garcia, R., Arrigada, R. (2003) A peach stone activated carbon chemically modifies to adsorb aqueous ammonia. Journal of the Chilean Chemical Society, 48(3), 235-241.
[https://doi.org/10.4067/S0717-97072003000300013]

-
Thote, J.A., Iyer, K.S., Chatti, R., Labhsetwar, N.K., Biwiwale, R.B., Rayalu, S.S. (2010) In situ enriched carbon for carbon dioxide capture. Carbon, 48(2), 396-402.
[https://doi.org/10.1016/j.carbon.2009.09.042]

-
Vehviläinen, T., Lindholm, H., Rintamäki, H., Pääkkönen, R., Hirvonen, A., Niemi, O., Vinha, J. (2016) High indoor CO2 concentrations in an office environment increases the transcutaneous CO2 level and sleepiness during cognitive work. Journal of Occupational and Environmental Hygiene, 13(1), 19-29.
[https://doi.org/10.1080/15459624.2015.1076160]

- Wachowski, L., Hofman, M. (2006) Application of TG-DTG analysis in the study of the ammoxidised carbon materials. Journal of Thermal Analysis and Calorimetry, 83(2), 379-383.
-
Wang, J., Adelodun, A.A., Oh, J.M., Jo, Y.M. (2020) TEPA impregnation of electrospun carbon nanofibers for enhanced low-level CO2 adsorption. Nano Covergence, 7, 1-11.
[https://doi.org/10.1186/s40580-020-0217-y]

-
Wang, J., Wang, M., Li, W., Qiao, W., Long, D., Ling, L. (2014) Application of polyethylenimine-impregnated solid adsorbents for direct capture of low-concentration CO2. American Institute of Chemical Engineers Journals, 61(3), 972-980.
[https://doi.org/10.1002/aic.14679]

-
Wu, X., Yuan, B., Bao, Z., Beng, S. (2014) Adsorption of carbon dioxide, methane and nitrogen on an ultramicroporous copper metal-organic framework. Journal of Colloid and Interface Science, 430, 78-84.
[https://doi.org/10.1016/j.jcis.2014.05.021]

-
Ye, Q., Jiang, J., Wang, C., Liu, Y., Pan, H., Shi, Y. (2012) Adsorption of Low-Concentration Carbon Dioxide on Amine- Modified Carbon Nanotubes at Ambient Temperature. Energy & Fuels, 26, 2497-2504.
[https://doi.org/10.1021/ef201699w]

-
Yu, J., Chuang, S.S.C. (2017) The Role of Water in CO2 Capture by Amine. Industrial & Engineering Chemistry Research, 56, 6337-6347.
[https://doi.org/10.1021/acs.iecr.7b00715]

-
Yuan, H., Meng, L.-Y., Park, S.-J. (2016) KOH-activated graphite nanofibers as CO2 adsorbents. Carbon Letters, 19, 99-103.
[https://doi.org/10.5714/CL.2016.19.099]