The Potential Contribution of VOCs on Ambient Air Odor
Copyright © 2021 by Asian Association for Atmospheric Environment
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In order to evaluate the potential contribution of VOCs on ambient air odor in roadside, urban, suburban and mountainous environments, the odor activity values (OAVs) of 66 VOCs were calculated using the environmental concentrations and the odor detection threshold values. The OAVs were less than one, but were not small, considering previous reports on odor concentrations in urban ambient air. The OAVs from roadside and urban areas were nearly identical, indicating that automobile exhaust gas does not have an important role in the potential odor. Acetaldehyde, which is directly emitted from anthropogenic sources and is also formed as a byproduct of photochemical reactions in the air, appears to be the most important substance contributing to the potential odor in each area. We also found that the contribution of biogenic VOCs was large in mountainous regions. In order to determine more precise OAVs, further work is required to generate more reliable odor detection threshold values.
Keywords:
Environmental odor, Odor concentration, Odor activity value, Odor detection threshold, Acetaldehyde1. INTRODUCTION
The ambient air from areas where there are no specific factories or industries has certain odors even though humans cannot perceive them clearly. Even if the odor concentration, (i.e. the number of dilutions with neutral air required to reach the odor detection threshold), is not measureable by normal dilution methods such as the triangle odor bag method (Iwasaki, 2003) or dynamic olfactometry (EN13725, 2003), it may still impact air quality.
The measurement of low odor concentrations in ambient air has been attempted in Japan. Tatsuichi et al. (1992) measured odor concentrations of 1.1-1.5 in residential suburbs and 7.4-9.8 in roadside areas utilizing the Japanese triangle odor bag method with preconcentration using three adsorbents and thermal desorption. These results demonstrated the contribution of vehicle exhaust gas to the odor concentrations. Tatsuichi and Ueno (1997) measured the odor concentrations in five districts in Tokyo. They also conducted a questionnaire survey of the residents, focused on the pleasantness of the air, which revealed that the residents found the air unpleasant if the odor concentration was above 2.5. Masuda et al. (2008, 2004) reported odor concentrations of 0.3-5.6 in Osaka city with higher odor concentrations at roadside areas. They utilized cryogenic condensation conjunction with the triangle odor bag method to quantify the odor concentrations. Cryogenic condensation effectively traps more odorous substances compared to the trapping by the absorbents.
Specific VOCs may contribute to low odor concentrations. Masuda et al. (2008) suggested that acetaldehyde was the most significant odor substance in the urban atmosphere according to the VOC concentrations and the odor detection thresholds (Nagata, 2003). Seo et al. (2014) measured VOC concentrations in the ambient air from industrial and residential areas of South Korea. They found that specific aldehydes had higher odor impact, based on the values calculated by dividing the observed concentrations with the standard odor intensity concentrations.
In this study, we examined the potential contribution of VOCs to odor in urban, roadside, suburban and mountain air in Tokyo. This analysis was performed by evaluating the odor activity values (OAVs) calculated based on the VOC concentrations and the odor detection threshold values. Since the VOC concentrations comprise the 24-hr average, the ambient air odor discussed in this study correspond to the background odor level of the area.
2. MATERIALS AND METHODS
2. 1 VOC Data
VOC concentrations in ambient air were obtained from the Bureau of the Environment, Tokyo Metropolitan Government (Bureau of the Environment, 2019, 2015). The data from Fiscal year (FY) 2013 and FY2017 were used, because the quality of the measurements, which depends on the analytical equipment, was consistent during these periods. VOC measurements with 24-hr sampling were performed once a month at 13 sites in Tokyo, including urban, suburban, roadside and mountainous areas (Fig. 1). Air samples were collected in a canister for most of the VOCs and analyzed by gas chromatography with flame ionization detection (GC-FID) for low volatile compounds (C2-C4 hydrocarbons), and gas chromatography-mass spectrometry (GC-MS) for other compounds. Aldehydes samples were collected with a 2, 4-dinitrophenyl hydrazine (DNPH) cartridge and extracted with acetonitrile followed by HPLC analysis. At each sampling point, air pollutants such as ozone and NOx were continuously monitored.
2. 2 Odor Detection Threshold
The odor detection threshold values used in this study were measured using the triangle odor bag method (Iwasaki, 2003). The following three Japanese institutes quantified the odor detection threshold values; the Japan Environmental Sanitation Center (Nagata, 2003), the Tokyo Metropolitan Research Institute for Environmental Protection (Iwasaki, 2004) and the Hiroshima Prefectural Research Center for Environmental Science (Matsusita et al., 1984; Ito et al., 1982). The odor detection threshold value depends on the sensitivity of the assessors who participated in the olfactory measurements. The three institutes used six different assessors. To reduce odor detection threshold value variability, we used the geometric mean in case of multiple data for each compound.
2. 3 OAV
The OAV is defined as the ratio of a compound’s concentration to the odor detection threshold. The OAV has been widely used to evaluate the relative contribution of odorous compounds to odor concentration (Rincón et al., 2019; Capelli et al., 2008). When only a single substance is present in the air, the OAV corresponds to the odor concentration. In the case of gas mixtures, the sum of individual OAVs (OAVsum) and the maximum OAV (OAVmax) can be used for odor assessment.
In this study, the OAVs of 66 VOC compounds (alkane and cycloalkane: 24, alkene: 3, biogenic: 5, aromatic: 15, aldehyde: 2, ketone: 3, oxygenate: 7, halide: 5, others: 2), which have the odor threshold values, were calculated. The compounds and the corresponding odor detection threshold values are listed in Table 1.
3. RESULTS AND DISCUSSION
3. 1 OAV
The OAVs (calculated from the annual average VOC concentrations) and NOx concentrations at each site as well as the corresponding mean values from the different areas are shown in Table 2. Both OAVmax and OAVsum values are less than 1, but not significantly small. Although there are no recent reports of ambient air odor concentration values, a previous study (Masuda et al., 2008) measured the odor concentration of ambient air using the triangle odor bag method with cryogenic preconcentration. They reported that the odor concentrations at 26 points in Osaka, Japan varied from 0.6 to 3.8. This report also found that the odor concentrations tended to be higher at roadside areas and lower at suburban regions. Osaka is a densely populated city, comparable to Tokyo, Japan. Considering the data, the OAVs values in Table 2 imply that the VOC odor is not clearly perspective by humans, but may contribute to the odor of ambient air.

Annual average OAVs and NOx concentrations at each site as well as the area corresponding mean values from the different areas.
Roadside area OAVs are nearly identical to the urban area OAVs as shown in Table 2. However, NOx concentrations from roadside regions are higher than those from urban areas, indicating that VOCs from automobiles have small direct effect on the OAVs. Previous studies (Masuda et al., 2008; Tatsuichi and Ueno, 1997) showed high odor concentrations at roadside areas. This suggests that the recent improvement of treatment systems for vehicle exhaust gas has effectively reduced the emission of odorous substances. On the other hand, the OAVs and NOx concentrations are the highest in urban regions followed by suburban areas and are the lowest in mountainous locations. This implies that higher total anthropogenic activity results in higher OAVs, although more data is required to substantiate this hypothesis.
Table 2 shows that site No.4 has the highest OAV, even though the NOx concentration is not especially high. In this area, there are many small factories which use organic solvents. This may explain the high OAV at the site No. 4 compared to other urban areas.
OAVs from 2017 are smaller than those from 2013 in all of the tested regions due to decreased VOC concentrations in ambient air. This was due to the combined efforts of various governments and industries whose goal was to reduce VOC and NOx concentrations in order to reduce ozone and particulate matter in the air. These efforts also contributed to the reduction in potential odor at all the sites shown in Table 2.
3. 2 VOC Compounds
The top ten VOC compounds from ambient air samples surveyed in 2017 were ranked according to their OAV and are shown in Fig. 2. Acetaldehyde was the largest contributor to the potential odor, comprising approximately 75% of the OAVsum in each area. The compounds composition in roadside and urban areas was nearly identical. Aside from acetaldehyde, there were butyl alcohols, butyl acetate and aromatic compounds such as toluene. These are common organic solvents that are widely used in many industries. Formaldehyde was observed as well as acetaldehyde. Several biogenic VOCs including α-pinene and isoprene were observed. The α-pinene OAV was larger in suburban and mountainous locations. The data indicate that biogenic VOCs potentially contribute to odor emissions in areas with less human activity, and may play a role in air quality.
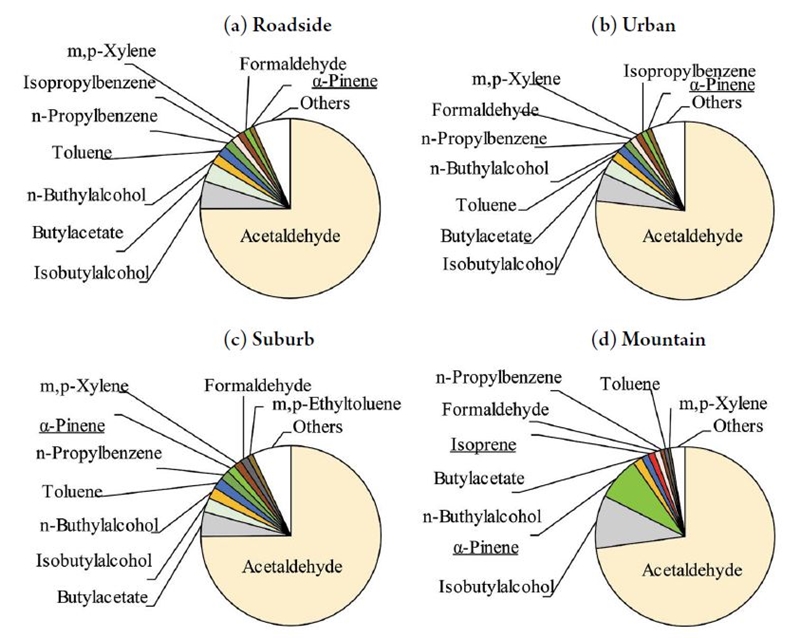
Top ten VOC compounds from an ambient air survey conducted in 2017 that are ranked according to their OAV. Underlined substances indicate biogenic VOCs.
The high acetaldehyde OAVs shown in Fig. 2 are due to their relatively high concentrations (2-4 ppb) and low odor detection threshold values [6.4 ppb as a geometric mean of 1.5 ppb (Nagata, 2003) and 27 ppb (Iwasaki, 2004)]. Masuda et al. (2008) also found that acetaldehyde had the largest OAV using the smaller threshold of 1.5 ppb. They suggested that the high acetaldehyde OAV was due to automobile exhaust gas because the odor concentrations at roadside areas were high and the maximum emission source of acetaldehyde was estimated to be automobile exhaust gas (MOE, 2005). According to the Pollutant Release and Transfer Register (PRTR), in 2017 (MOE, 2017) the estimated emission of acetaldehyde from mobile sources in Tokyo was 113 t/year, while that from households was 36 t/year and that from stationary sources was only 2.9 t/year. However, in our studies, the OAVs at roadside locations were not significantly higher than those from urban residential areas (Table 2). Moreover, NOx concentrations at roadside locations were significantly higher than those at urban areas (Table 2). Therefore, these OAVs may not only be due to automobiles, but may also originate from the following (1) industrial activity (which is not estimated in the PRTR); (2) secondary formation of acetaldehyde in the air (Seo et al., 2014) or (3) unreliable odor detection threshold values (Masuda et al., 2008).
Ishii et al. (2014) suggested that formaldehyde emission in Tokyo originated from combustion-related and non-combustion-related facilities such as boiler or wood factories. Acetaldehyde may also be emitted from unintentional sources. For example, in painting facilities, acetaldehyde is emitted during the drying process. Some amount of acetaldehyde may not be fully estimated by the PRTR.
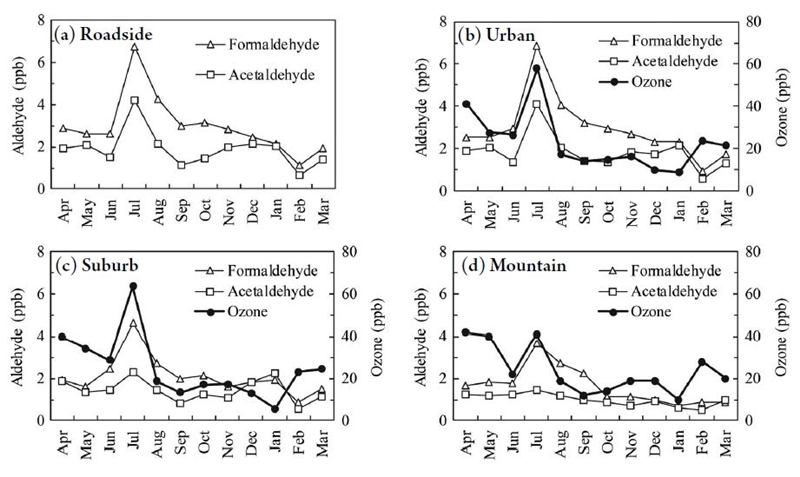
Aldehydes are known to be byproducts of photochemical reactions in the air (Sakamoto et al., 1993). Fig. 3 shows monthly data of formaldehyde, acetaldehyde and ozone concentrations from FY2013 during a typical summer peak of ozone was observed. In July, aldehydes and ozone concentrations were high. Ozone is produced by photochemical reactions, providing further evidence that aldehydes are also byproducts of photochemical reactions. According to the data from suburban and mountainous areas, formaldehyde is more likely to be formed than acetaldehyde. This tendency is consistent with a previous study (Ling et al., 2016). Taken together, these results imply that acetaldehyde is directly emitted from industrial sources and is also formed as a byproduct of photochemical reactions.
3. 3 Reliability of the Odor Detection Threshold
The OAV calculation is based on the assumption that the odor detection threshold is reliable. There are several compilations of odor detection thresholds. Ruth (1986) and U.S. EPA (1992) reported acetaldehyde odor detection thresholds of 0.1-2200 ppb and 6.7-390 ppb, respectively. It is difficult to use these numbers for the assessment of odorous substances because of the significant variation of three or five orders of magnitude. The discrepancy is due to the variability in both the measurement methods and human olfactory responses (Cain and Schmidt, 2009). Different institutes measured the odor threshold values in this study but they all used the same method. Fig. 4 shows the correlations of odor detection threshold values. There are good correlations in the double logarithmic plots. However, there are one order of magnitude differences in some compounds. The odor detection thresholds of acetaldehyde are 1.5 ppb determined by the Japan Environmental Sanitation Center (A) and 27 ppb determined by the Tokyo Metropolitan Research Institute for Environmental Protection (B). In this study, we utilized the geometric mean calculated from the measurements of the acetaldehyde odor detection threshold by A and B, which was found to be 6.4 ppb. If 27 ppb is used for the calculation of the OAV, the ratio of approximately 75% for acetaldehyde shown in Fig. 2 changed to approximately 40%, suggesting that acetaldehyde is still the most important substance contributing to potential odor in ambient air.
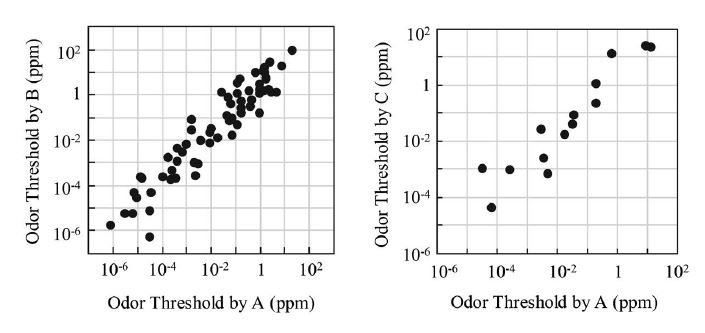
Comparison of odor detection threshold values measured by three institutes. A: Japan Environmental Sanitation Center (Nagata, 2003); B: Tokyo Metropolitan Research Institute for Environmental Protection (Iwasaki, 2004) and C: Hiroshima Prefectural Research Center for Environmental Science(Matsusita et al., 1984; Ito et al., 1982).
These odor detection threshold values were mainly measured in 1980s. After the 1990s, quality control of both sensory and instrumental analyses has been improved through amendments of the Japanese Offensive Odor Control Law and the Japanese Air Pollution Control Act. Thus, the reliability of the odor detection threshold values should be ensured by acquiring the data from quality-certified analytical institutes.
3. 4 Other Compounds
There may be other compounds that potentially contribute to the odor of ambient air. Sulphur compounds are possible candidates, due to their low odor detection threshold values. High concentrations of hydrogen sulfide were reported in a few urban environments in Greece (Kourtidis et al., 2008). Masuda et al. (2008) measured sulphur compounds at 26 urban locations in Osaka city. They reported that the OAVs were not high compared to the acetaldehyde OAVs except for a few areas, one of which was close to a sewage treatment plant. The effects of sulphur compounds on potential odor are not so high in Tokyo because environment is similar to Osaka. However, compounds with low odor detection thresholds should be taken into account in this kind of research.
4. CONCLUSIONS
VOC OAVs calculated by dividing the environmental concentrations with odor detection thresholds imply that VOCs might contribute to the odor of ambient air. Acetaldehyde, which is not only emitted from anthropogenic sources but also produced secondarily in the air, appeared to be the most important substance potentially contributing to odor of ambient air. In addition, biogenic VOCs may have important roles in air quality in mountainous areas. To assess these issues more precisely, accumulation of odor detection threshold values measured by quality-certified analytical institutes is necessary.
Acknowledgments
This work was supported by Japanese Ministry of the Environment.
References
- Bureau of the Environment, Tokyo Metropolitan Government (2015) Monitoring report of hazardous air pollutions in FY2013, Tokyo, Japan (in Japanese).
- Bureau of the Environment, Tokyo Metropolitan Government (2019) Monitoring report of hazardous air pollutions in FY2017, Tokyo, Japan (in Japanese).
-
Cain, W.S., Schmidt, R. (2009) Can we trust odor databases? Example of t- and n-butyl acetate. Atmospheric Environment, 43(16), 2591-2601.
[https://doi.org/10.1016/j.atmosenv.2009.02.024]

-
Capelli, L., Sironi, S., Del Rosso, R., Centola, P., Il Grande, M. (2008) A comparative and critical evaluation of odour assessment methods on a landfill site. Atmospheric. Environment, 42(30), 7050-7058.
[https://doi.org/10.1016/j.atmosenv.2008.06.009]

- EN13725 (2003) Air Quality - Determination of odor concentration by dynamic olfactometry. Committee for European Normalization (CEN), Brussels, Belgium.
- Ito, T., Matsushita, K., Ito, T., Kodama, M. (1982) Studies on olfactory measurement of odor IV - Measurements of threshold value by the triangle odor bag method - . Bulletin of Hiroshima Prefectural Research Center for Environmental Science, 4, 7-10 (in Japanese).
- Ishii1, K., Matsumoto, Y., Ito, M., Ueno, H., Uchida, Y., Saito, S., Hoshi, J., Nakashima, Y., Kato, S., Kajii, Y. (2014) Source attribution of high concentration peak events of formaldehyde in the central Tokyo metropolitan area. Journal of Japan Society for Atmospheric Environment, 49(6), 252-256.
- Iwasaki, Y. (2003) The history of odor measurement in Japan and triangle odor bag method, In: Odor Measurement Review, Office of Odor, Noise and Vibration Environmental Management Bureau, Ministry of the Environment, Tokyo, Japan, pp. 37-47. https://www.env.go.jp/en/air/odor/measure/index.html, (last accessed on Jul. 17, 2020).
- Iwasaki, Y. (2004) Syuki no Kyukaku Sokuteiho/Sensory method by human olfaction, Japan Association on Odor Environment, Tokyo, pp. 129-136 (in Japanese).
-
Kourtdis, K., Kelesis, A., Petrakakis, M. (2008) Hydrogen sulfide (H2S) in urban ambient air. Atmospheric Environment, 42(32), 7476-7482.
[https://doi.org/10.1016/j.atmosenv.2008.05.066]

-
Ling, Z., Guo, H., Chen, G., Lam, S.H.M., Fan, S. (2016) Formaldehyde and acetaldehyde at different elevations in mountainous areas in Hong Kong. Aerosol and Air Quality Research, 16(8), 1868-1878.
[https://doi.org/10.4209/aaqr.2015.09.0571]

-
Masuda, J., Fukuyama, J., Tonoike, M., Yamaguchi, M. (2004) Cryogenic trapping for determination of odor concentration. Water Science and Technology, 50(4), 121-124.
[https://doi.org/10.2166/wst.2004.0238]

-
Masuda, J., Itano, Y., Fukuyama, J. (2008) Odor concentration of ambient air and odor substances in Osaka city and its suburbs. Journal of Japan Association on Odor Environment, 39(1), 10-16.
[https://doi.org/10.2171/jao.39.10]

- Matsushita, K., Ito, T., Ito, T., Seto, N. (1984) Measurement of threshold value by trianglar odor bag method (II), The Proceedings of the 26th Annual Meeting of the Japan Society of Air Pollution, Tokyo, 400 (in Japanese).
- MOE (Ministry of the Environment, Japan) (2005) Chemical Substance Fact Sheets, Tokyo, Japan (in Japanese).
- MOE (Ministry of the Environment, Japan) (2017) Pollutant Release and Transfer Register Data, http://www2.env.go.jp/chemi/prtr/prtrinfo/contents/2017/html_jp/T3_2017999999.htm, (accessed on 23 Sep. 2020).
- Nagata, Y. (2003) Measurement of odor threshold by triangular odor bag method. In: Odor Measurement Review, Office of Odor, Noise and Vibration Environmental Management Bureau, Ministry of the Environment, Tokyo, Japan, pp.118-127, https://www.env.go.jp/en/air/odor/measure/index.html, , last accessed on Jul. 17, 2020.
-
Rincón, C.A., Guardiaa, A.D., Couvertb, A., Wolbertb, D., Rouxa, S.L., Soutrelb, I., Nunesa, G. (2019) Odor concentration (OC) prediction based on odor activity values (OAVs) during composting of solid wastes and digestates. Atmospheric Environment, 201(15), 1-12.
[https://doi.org/10.1016/j.atmosenv.2018.12.030]

-
Ruth, J.H. (1986) Odor Thresholds and irritation levels of several chemical substances: A review. American Industrial Hygiene Association Journal, 47(3), A-142-A-151.
[https://doi.org/10.1080/15298668691389595]

-
Sakamoto, K., Kojima, T., Hara, S., Otsuka, S., Iwamoto, I., Yamaki, N. (1993) Primary emission and secondary formation of aliphatic and aromatic aldehydes in the summer season’s ambient air. Journal of Environmental Chemistry, 3(4), 729-737.
[https://doi.org/10.5985/jec.3.729]

-
Seo, Y.-K., Suvarapu, L.N., Baek, S.-O. (2014) Characterization of odorous compounds (VOC and carbonyl compounds) in the ambient air of Yeosu and Gwangyang, Large industrial areas of South Korea. The Scientific World Journal, 2014, Article ID 824301.
[https://doi.org/10.1155/2014/824301]

- Tatsuichi, S., Iwasaki, Y., Ueno, H. (1992) Study on measurement of environmental odor by sensory test, Annual Report of the Tokyo Metropolitan Research Institute for Environmental protection, 1992, 9-14 (in Japanese).
- Tatsuichi, S., Ueno, H. (1997) A study of the environmental odor in Tokyo. Annual Report of the Tokyo Metropolitan Research Institute for Environmental protection, 1997, 207-215 (in Japanese).
- U.S. EPA (1992) Reference guide to odor thresholds for hazardous air pollutants listed in the clean air act amendments of 1990, U.S. Environmental Protection Agency, Washington, D.C., EPA/600/R-92/047.