Recent Advances in Biochar-based Catalysts: Air Purification and Opportunities for Industrial Upscaling
Abstract
The preparation of eco-friendly carbon-rich (biochar) materials by thermal pyrolysis of waste biomass has been recognized as one of the most economical and effective strategies for gas purification in recent years. Through control of synthesis and activation methods, the surface features and catalytic sites in biochar can be engineered for diverse heterogeneous catalytic reactions. Nonetheless, its commercial utilization in air pollution control has yet been limited to a large extent because of (i) the shortage of databases related to the actual catalytic performance of biochar and (ii) the complexity involved in industrial upscaling. Herein, the merits and demerits of biomass-to-biochar catalyst conversion are discussed, along with the factors to consider in the synthesis stage for enhancing catalytic activities toward air purification applications. This paper also offers an in-depth evaluation of the techno-economic and environmental aspects of biochar-based catalysts and their catalytic reactions for air pollution control and energy production. Lastly, a contemporary perspective is offered to help develop novel biocharbased catalysts for real-world applications in air purification fields.
Keywords:
Biochar catalysts design, Heterogeneous catalysis, Air purification, Energy applications, Industrialization challenges, Sustainability1. INTRODUCTION
The concept of waste-to-energy (WtE) is a smart technique used globally for waste management with a beneficial impact on both the economy and the environment. In particular, the gasification/pyrolysis of biomass wastes (as renewable resources) to generate heat, fuels (e.g., biogas, biodiesel, and bioethanol), or value-added economic products have attracted a good deal of research interest worldwide (Guo et al., 2020; Balajii and Niju, 2019).
In the context of renewable resources, biomass materials include a variety of macromolecular organic matters (e.g., agriculture wastes, forest woods, sewage, and food products) with carbon, oxygen, and hydrogen as major elemental constituents. In the last five years, the global annual production of waste biomass was approximately 140 Gt, in which 580, 451, 682, 605, and 716 Mt (fresh weight)/year were generated by the European Union (EU) member states, Brazil, USA, India, and China, respectively (Casson Moreno et al., 2020; Tripathi et al., 2019). Such an issue is a significant management problem as it has high negative impacts on the economy and environment (e.g., >2 Gt waste residue is burned, accounting for 18% of global CO2 emissions). The direct/indirect conversion of biomass to bioenergy (or WtE) has been an appealing option to manage this problem, as it can account for an approximate share of 96%, 59%, 65%, and 65% of total primary energy supplies in 2019 for Africa, USA, EU, and Asia, respectively, based on global bioenergy statistics (GLOBAL BIOENERGY STATISTICS-World Bioenergy Association, 2019). However, it should be noted that two challenges are facing the current WtE management protocols: (i) environmental pollution like the high emission of greenhouse gases (GHGs) like CO2 (>47 Mt/year) and generation of high quantities of particulate ash/black carbons (Tripathi et al., 2019) and (ii) high capital cost for the installation of WtE power plants (400 to 6,100 USD/kW), depending on the feedstock biomass cost, type of technology, and country/region (Ghosh, 2016). Hence, it is essential to maximize the structure/efficiency of WtE power plant technologies to reduce CO2 emission and bioenergy production costs at the same time. In this respect, the generated ash/black carbon residues are can be utilized as sustainable carbon-negative products to capture point-source CO2 produced during the incineration process. The capital cost of bioenergy production can also be reduced by using sustainable, low-cost feedstock that can be generated onsite (such as black liquor in the papermaking industry) or transported over short distances (e.g., agriculture waste-byproducts collected from nearby farmland).
Thermo-chemical conversion of biomass wastes to biochar (BC) has become one of the most sustainable and effective protocols for the management/recycling of waste biomass in the last decade (Fig. 1). BC is a low-cost, sustainable, and eco-friendly porous carbon-rich solid with high chemical stability, low bulk density, tunable electrical/photonic responses, and abundant surface oxygen functional groups (OFGs; e.g., OH, C=O/C-O, and COOH) (Do Minh et al., 2020). The main constituents of BC are carbon, hydrogen, oxygen, and ash, with quantitative ranges of 60-89% (fixed aromatic carbon≈10.7-86.4%), 1-7%, 9-36%, and 0.2-40%, respectively. BC products also contain a good number of inorganic mineral elements (e.g., N, S, P, Na, K, Ca, Mg, Fe, Zn, Cd, As, Se, and Si) that exist as micronutrients in the raw feedstock biomass (Chen et al., 2019; Liu et al., 2015). The morphological structure of BC products is turbostratic graphite-like layers with porous networking structures. These inherent features of BC are strongly dependent on the raw biomass sources, activation methods, and pyrolysis conditions (e.g., characteristic features of yielded products). Accordingly, the recent progress in the synthesis and application of BC-based materials has been recognized in various fields (Fig. 1) such as agro/environment (Shaheen et al., 2022; Younis et al., 2021), adsorption/separation for environmental contaminants (Lee et al., 2018; Xiong et al., 2017), electrochemistry (Guo et al., 2020), heterogeneous catalysis (Lee et al., 2017), photocatalysis (Mian and Liu, 2018), air pollution control (Fawzy et al., 2021; Gwenzi et al., 2021, CO2 capture and utilization (CCU) technologies (Guo et al., 2022; Zhang et al., 2022c), and energy storage/conversion (Bolan et al., 2022; Liu et al., 2019).
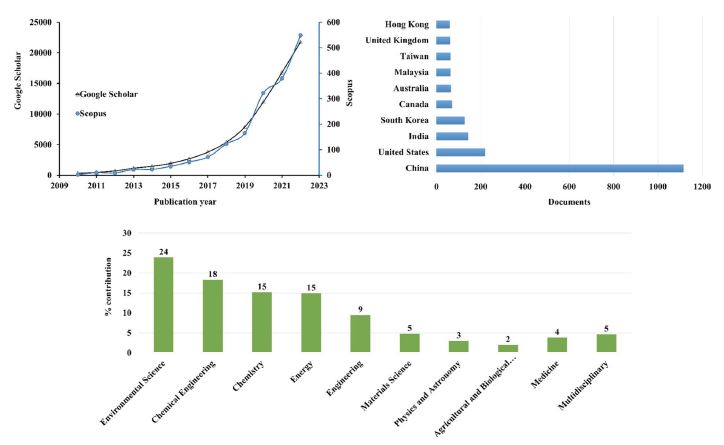
Meta-analysis of the research databases (Google scholar and Scopus) available for publications with “Biochar” and “Biochar catalyst” as keyword indexes over the last decade (2010-2022).
In particular, the development of BC-based catalysts has received global attention in the fields of biodiesel production, refinery (syngas cleaning/conversion), and air/water pollution control (Bolan et al., 2022; Younis et al., 2020). Recently, several research studies have been published to help boost the utilization of BC in those mentioned heterogeneous catalytic applications (Wang et al., 2019; Cheng and Li, 2018; Xiong et al., 2017). According to reports made by Grand View Research statistics (Size, 2020) and BCC Research (McWilliams, 2015), the global market for catalysts in environmental and energy fields is projected to reach $35.8 billion in 2020, with an annual growth rate of 4.4% to 2027. This is due to the key role of catalysts in developing technologies to generate value-added chemical (or biorefinery) products and to control environmental pollution. Despite the potential utility of BC, most recent studies have been directed to the following key parameters: (i) the synthesis or post-synthesis modification strategies to derive BC-based catalysts and (ii) the interrelations between the physicochemical properties of BC materials and their catalytic performances in target catalytic reactions (Anto et al., 2021; Do Minh et al., 2020; Shan et al., 2020). However, it was found that the techno-economic/fate-life cycle assessments of BC-based catalysts were rarely addressed when evaluating production scale-up (e.g., from lab- to industrial-scale) for solving global energy and environmental problems. To help expand our knowledge in this research field, the potential upgrade of BC-based catalysts as alternative low-cost commercial catalysts in environmental applications is discussed by highlighting: (i) biochar-based materials for air pollution control, (ii) the factors involved in the synthesis of BC catalysts and production scale-up, (iii) the pros and cons of BC-catalysts in heterogeneous catalysis (relative to traditional metal-based catalysts) for CCU technology, and (iv) the fate-life cycle and techno-economic aspects of BC catalysts during manufacturing and in applications.
2. ADVANCES IN BIOCHAR-BASED MATERIALS FOR AIR PURIFICATION
2. 1 Mitigation of Volatile Organic Compounds (VOCs)
To date, biochar is considered one of the most commercially viable porous carbon materials in environmental pollution control with the aid of its unique physicochemical features (e.g., large surface area with good porosity, high cation exchange capacity (CEC), and high thermochemical stability). It is widely used as an effective adsorbent for the control of the emission of VOCs to the indoor/outdoor environment based on the two key mechanisms: adsorption and partitioning in carbonized/non-carbonized organic regimes, respectively (Yue et al., 2021; Zhang et al., 2017), as shown in Fig. 2. Likewise, the adsorption mechanism of VOCs over biochar could involve chemical (e.g., covalent and H-bondings) and/or physical mechanisms (e.g., dipole, Π, columbic, and hydrophobic interactions), depending on the interactive relationship between physiochemical properties of biochar and the nature of VOC adsorbates.
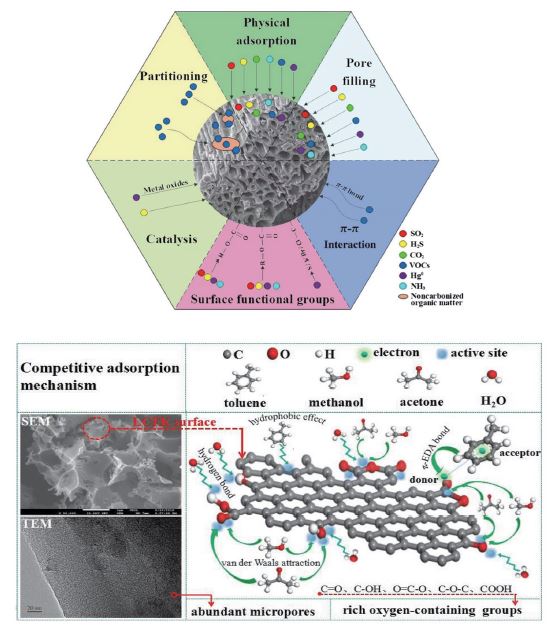
The contribution of oxygen-containing surface functionalities and porosity of carbon adsorbents like biochar in the adsorption mechanisms of VOCs (Zhao et al., 2022; Meng et al., 2019).
The applicability of biochar for the enhanced adsorption removal of VOCs from the atmosphere and/or gas flue is determined by (i) the factors affecting its textural (porosity), morphological, and chemical features (O/C and H/C molar ratios) such as the raw feedstock, pyrolysis/synthesis conditions, and surface modification and (ii) the operational parameters for VOCs adsorption (e.g., target VOCs, air/gas composition, bed temperature, bed height, humidity, and flow velocity). It should be noted that the O/C and H/C molar ratios could control the adsorption selectivity of biochar for targeted VOCs as they refer to the polarity and quantization aromaticity levels of biochar. For example, altering the feedstocks could significantly affect the surface porosity and surface area of prepared biochars, even if they are synthesized at the same pyrolysis temperature. In this regard, the derived biochars from pine wood, hickory, cotton, corn straw, alfalfa, wheat straw, switchgrass, and rice husk at 600°C had different surface areas of 312, 256, 2.2, 61, 0.2, 182, 15, 168 m2 g-1, respectively (Yue et al., 2021). An increased surface area and porosity could help biochar adsorbents to enhance the capacity for GHGs adsorption via the pore diffusion mechanism (Thangarajan et al., 2018). Altering the feedstock from corn straw to rice husk was reported to change the aromaticity level (H/C molar ratio) of derived biochar from 0.002 to 0.04. On the contrary, the biochar derived from switchgrass showed the highest surface polarity (O/C molar ratio of 0.37) compared with that of hickory wood biochar (O/C=0.03) (Uchimiya et al., 2010; Abe et al., 2000). The surface polarity of biochar also decreased with a rise in the pyrolysis temperature due to the accelerated removal of oxygen-containing functional groups (OCFGs) (Monga et al., 2022). As the surface polarity decreased, the aromaticity increased to promote the adsorption potential of hydrophobic VOCs. For instance, the effects of physicochemical properties on the adsorption applicability of different biochars (n=15) were assessed against varying types of polar and nonpolar VOCs (e.g., acetone, ethanol, methanol, cyclohexane, hexane, benzene, xylene, and toluene) (Yue et al., 2021). Accordingly, an interactive relationship between surface area and the ratio of carbonized/non-carbonized organic matter was observed to be crucial to control their adsorption property against diverse VOCs. In particular, the adsorption affinity of biochar for polar VOCs (e.g., acetone) was maximized (capacity=483.09 mg g-1) via promoting pore diffusion and physical interactions in the adsorption mechanism along with increases in carbon pore structure and in non-carbonized organic matter (e.g., increase in O/C ratio) (Li et al., 2012).
Among air pollution control strategies, the utilization of biochar to sequester and mitigate CO2 in soils is well-reported in many reviews (Smith, 2016; Thomazini et al., 2015). However, it was noted that the sorption capacity of pristine biochar for CO2 was low via a weak physisorption mechanism (e.g., van der Waals forces). The affinity of biochar for CO2 capture is greatly dependent on its physiochemical features such as surface porosity, hydrophobicity, aromaticity, and basicity (i.e., the content of Lewis base active sites). These physicochemical features of biochar could be adjusted by controlling the pyrolysis and/or synthesis conditions as well as the type of feedstock (Gwenzi et al., 2021). Because of the low CO2 affinity of pristine biochar, numerous studies have been focused on engineering its surface features (e.g., doping with metal oxides and heteroatoms) to improve CO2 sequestering potential for industrial applications (i.e., from flue gases at high-temperature conditions). For instance, doping biochar with a basic nitrogen group was carried out to accelerate the uptake rate of CO2 by approx. 55% relative to bare biochar (Xu et al., 2019).
In light of the great potential of metallic constituents of biochar in the chemisorption of CO2 and its transformation reaction, the catalytic oxidation/reduction reactions of such biochar-based composites have been studied against GHGs (like NOx, SOx, CO2, and VOCs) under thermal and nonthermal (photo and plasma) conditions (Xu et al., 2016). As such, compared to pristine biochar, engineered biochar-supported metal oxides have attracted great research interest as catalysts with enhanced chemisorption capacity for GHGs (e.g., CO2) (Gwenzi et al., 2021). A number of chemical and physical activation/modification methods have been applied to improve the catalytic efficiency of biochar catalysts in mitigating VOCs/GHGs emissions as a prospective solution to control the climate shift (Abhishek et al., 2022; Do Minh et al., 2020; Kumar et al., 2020c).
As a good example, the intercalation of bone char with ZnO nanoparticles (nano-ZnO NPs) was reported to improve their photocatalytic activity towards the formaldehyde (2.5-25 mg m-3) to attain 75.5% of removal from the humidified indoor air (at 35% relative humidity) under ultraviolet light irradiation (Rezaee et al., 2014). At higher humidity levels, the photocatalytic decomposition of formaldehyde by nano-ZnO@bone char decreased due to the competitive inhibition effects of water vapor for adsorption sites and a downward band bending of ZnO to increase the recombination rate of photogenerated charge carriers (i.e., the photoactivity decreased). A synthesized composite of biochar with g-C3N4 nanosheets by co-thermal pyrolysis of melamine and cellulose precursors also accelerated the photocatalytic removal rate of formaldehyde by 130% higher than that of pristine g-C3N4 nanosheets “Chrysanthemum” under visible light irradiation (Li et al., 2019). Such observation was accounted for by the fact that the high electron storage capacity of biochar skeleton should be imparted by the π-conjugation system (as an effective electron acceptor) and improved charge carriers separation capacity (i.e., extended lifetime of excited electrons). In another study, doping a sawdust char (SDW) with MnOx nanocatalyst was carried out to accelerate the oxidative destruction of toluene (89.7% after 100 min) (Cha et al., 2022). The enhanced catalytic activity of MnOx/SDW composite was accounted for by the high redox potential of Mn3+ and the abundance of Ov (vacancy oxygen))/OL (lattice oxygen) generated on the SDW surface upon doping. In summary, although some VOCs might release into the atmosphere during biochar formation, the engineered biochar with high adsorption/catalytic properties appears to be a promising candidate for the effective control of GHGs and VOCs emitted from industrial sectors (flue gases).
2. 2 CO2 Capture and Utilization (CCU Technology)
The chemical activation and doping of biochar (BC) with metal oxides played vital roles in CCU reactions (catalytic conversion of CO2 to renewable fuels and other chemicals). Hence, over the last decade, the utility of multiple chemical agents has been investigated for the activation of BC materials, such as KOH, KHCO3, and amine agents (Zhang et al., 2022a; Chatterjee et al., 2020, 2019; Ding and Liu, 2020). As the first step in the CCU technology involves CO2 capture, these chemical activations were used to improve the textural features (microporosity and surface area) and surface functionalities (ie. g., surface basicity and Lewis base sites) of BC materials for the enhancement of adsorption capacity toward CO2. In the second step of CCU technology, the catalytic activity of BC materials is mainly considered with respect to the metal contents and properties (e.g., oxidation state, dispersion patterns, and morphological features) (Shrestha et al., 2022).
In this respect, doping BC with metal oxides is a growing area of research interest for developing sustainable and cost-efficient catalysts from waste sources for the ultimate applications toward selective catalytic CO2 reduction reactions (CO2-RR). For example, biochar-ZnO composite has been prepared to have a stable and selective electrocatalytic activity in the CO2-RR to CO (85.8%) at -1.09 V versus the RHE in a batch reactor (Lourenço et al., 2021). Likewise, in light of the high carbon and energy density, biochars have been used as reducing agents to enhance CO2-RR into O2-free fuel gas (21.3-27.1%) in an atmospheric plasmatron via the Boudouard reaction (CO2+C → 2CO, ΔH=172 kJ mol-1) (Zhang et al., 2022b; Huang et al., 2021). It is noted that the contribution of Boudouard reaction (i.e., in the presence of biochar) in the overall CO2-RR under an atmospheric plasmatron is approx. >96.0%. Accordingly, the use of biochar in CO2 splitting processes via Boudouard reaction in non-thermal plasmas could offer a more efficient route for the effective purification of industrial flue gases. Because the direct thermocatalytic reduction of CO2 (CO2 → CO+1/2O2, ΔH=280 kJ mol-1) requires high thermal energy conditions(> 850°C at 1 atm) that can limit its flexibility of the scale.
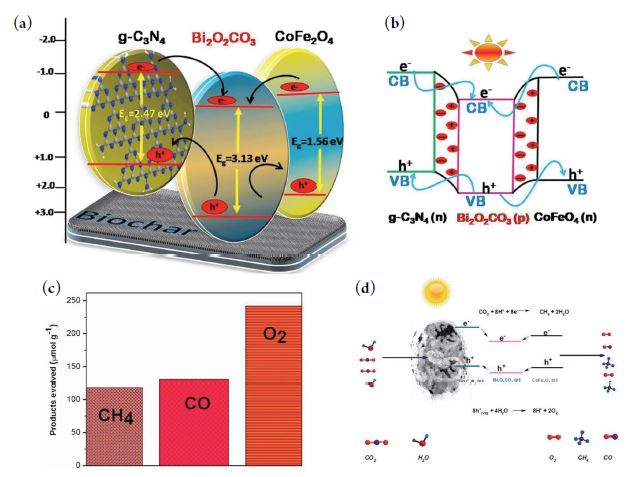
The photocatalytic CO2-RR over BC-based catalysts has also attracted research interest as one of the effective, inexpensive, and environmentally friendly strategies for CO2 conversion to value-added solar fuels (e.g., HCO2H, CH2O, CH4, and CH3OH) under solar irradiation condition (Bhavani et al., 2022; Lin et al., 2022; Rangarajan et al., 2022). Hence, several types of BC-based composite photocatalysts have been introduced in recent years to study their role in boosting CO2-RR pathways. For instance, the developed BC-templated g-C3N4/Bi2O2CO3/CoFe2O4 composite (BCBF) heterojunction showed promising photocatalytic activity for CO2-RR into CH4 (∼119 μmol g-1) and CO (∼131 μmol g-1) in 8 h under visible light irradiation (Kumar et al., 2018). The boosted photocatalytic activity of BCBF is attributable to the uniform dispersion of Bi2O2CO3/CoFe2O4 nanocatlysts with low bandgap energies (i.e., solar light harvester), high capability of g-C3N4 (with lewis base sites) for CO2 capture onto the catalytic surface, and the mediator role of BC template in reducing the electrons-holes (e-/h+) recombination rate (Fig. 3). In another study, the Ag-g-C3N4/biochar nanocomposite with a hollow bird’s nest-like carbon (BN-C) structure was prepared with a selective photocatalytic CO2-RR to CO at a production rate of 33.3 μmol h-1 g-1 which was far higher (e.g., by around three folds) than the pristine g-C3N4 counterpart (CO production rate of approx.. 10.3 μmol h-1 g-1) under visible light (Li et al., 2022). In this case, the high photocatalytic activity of the former was assigned to the localized surface plasmon resonance (LSPR) effect of Ag NPs to endow an efficient utilization of solar light energy and to produce more active “hot electrons. In addition, the co-existence of Ag NPs and conductive BN-like carbon structure may impart more surface active sites to enhance CO2 capture, electron trap states, and charge separation efficiency, which, in turn, accelerates photo-induced electrons of g-C3N4 photocatalyst with great potential for the CCU process. Multiple Cu-doped hydrochar (Cu-HTCC) photocatalysts were also developed by hydrothermal carbonization (HTCC) of different biomass feeds (such as rice, straw, glucose, and cow dung) in the presence of CuSO (Hu and Liu, 2020). The developed Cu-HTCC photocatalysts exhibited an outstanding performance for catalytic conversion of CO2 to CO, with a production rate of 564.8 to 643.5 μmol h-1 g-1, which is superior to the above-mentioned BCBF catalyst and 32-time higher than commercial P25-TiO2 photocatalyst. The improved photoactivity of Cu-HTCC was accounted for by the HTCC sp2-hybridized structures that promoted catalytic reduction of CO2 reduction with the enhanced solar light harvesting capacity (e.g., over Cu co-catalyst).
3. FACTORS AFFECTING BIOMASS-TO-BIOCHAR CONVERSION FOR CATALYTIC APPLICATIONS
Although the source materials and production methods of biochar are similar to activated carbon (AC), their distinctions are apparent. For example, the production temperature of biochar (e.g., less than 700°C) is much lower than that of AC (e.g., up to 900°C) (Zhang et al., 2017). Besides, the process of activation is unnecessary during biochar production, while it is crucial for AC production. In addition, the break-even price of biochar is about US $246/ton, which is only 1/6 of that for commercial AC (Awasthi, 2022; Amusat et al., 2021).
At present, several thermo-chemical methodologies have been reported for the development of BC from biomass wastes such as slow/fast pyrolysis, hydrothermal carbonization, microwave-assisted heating, gasification, rectification, and torrefaction methods (Guo et al., 2020; Qian et al., 2015). Briefly, the slow pyrolysis method (e.g., low temperature (at 350-600°C) for long residence times (hours to days) in oxygen-limited conditions) is the most widely used method for BC production with specific surface areas in the range of 1.5 to 241 m2 g-1 (Kumar et al., 2020a; Wang et al., 2019). Chemical activation of raw biomass is also commonly used before thermal pyrolysis to control Brønsted-to-Lewis acid/base ratios (using H2SO4, KOH, NaOH, or H3PO4) or to anchor catalytic sites (using NiCl2, ZnCl2, Sn(OH)4, Co(OH)2, or MnCl2) for target catalytic reactions. This method is also adopted to improve the physicochemical features of BC materials (e.g., increases in the swelling/hydrolysis of lignocellulosic contents) before thermal pyrolysis. In this case, the BC products should show improved texture properties (e.g., porosity, pore volume, and surface area), high aromaticity (e.g., greater fixed carbon and lower crystallites), and low content of undesired components (e.g., ash or volatile carbon). For instance, the adjustment of KOH: biomass char ratios from 1 : 1 to 1 : 3 can increase the surface area (from 853 to 1686 m2 g-1) and pore volume (from 0.34 to 0.64 cm3 g-1) to favorably improve catalytic reactions (Cha et al., 2016). Similarly, hydrothermal treatment and/or oxygenation of raw biomass can also be used to increase the texture (e.g., surface area and porosity) properties of BC products.
In typical pyrolytic conditions (at <500°C for 12 h or >500°C for 1 h, with a yield of 30-40%), about 700 kg of BC can be synthesized from the pyrolysis of two tons of biomass (Liu et al., 2015). This shows that BC can be scaled-up for practical applications in multiple fields as catalysts. However, it should be noted that the catalytic activities of pristine BC materials (without pre- or post-synthesis modification) are rarely reported, especially in photocatalysis and thermocatalysis. This is mainly due to the absence or deactivation of inherent catalytic sites (Brønsted acid/base or semiconducting metal sites) during the pyrolytic synthesis of BC. For example, the catalytic capacity of BC could dramatically decrease when using a high pyrolytic temperature (>volatilization temperature of minerals) for longer reaction times during thermochemical synthesis due to the gradual cracking of phenolic (e.g., quinone/hydroquinone) constituents and to the deactivation/volatilization of inherited metallic sites associated with catalytic activity in BC (Lyu et al., 2020). Further, the utilization of feedstock biomass with a high content of mineral elements can also significantly decrease the stability and electrical conductivity of BC, which is required to improve heterogeneous photocatalytic activity. This observation is attributed to the destruction of conjugated aromatic carbon or preventing its formation in the presence of high mineral content (like Si) during pyrolytic synthesis (McBeath et al., 2015). These findings suggest that the catalytic efficiency of BC is dependent on its physicochemical properties (surface area, porosity, hydrophobicity, and surface functionality) and the availability of inherent catalytic sites (Brønsted acid/base sites or semiconducting metal sites). It is thus essential to understand the fundamentals associated with the production of BC to develop BC catalysts with promising performance in target catalytic reactions.
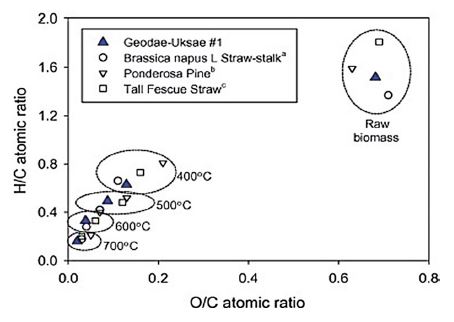
There are many contrasting factors to consider for controlling BC catalytic activity (Kumar et al., 2020a; Xiong et al., 2017). For instance, the use of a feedstock with high lipid/ash content can lead to a reduction in aromaticity, porosity, and surface area (unfavored) of the produced BC. On the other hand, biomass with rich lignin/cellulose content increases porous graphitic structure and carbon stability (favored for catalysis). Notably, the weight ratios of water to biomass in hydrothermal carbonization are found to have a direct effect on the catalytic capacity of BC materials. This is due to the significant role of water in the pyrolysis of biomass and the formation of persistent free radicals (PFRs: e.g., sulfate (SO4●-) or hydroxyl (HO●)) on the BC surface (Lyu et al., 2020). Note that the concentration of PFRs is dependent on the metal and phenolic content in BC. In this context, the use of high water: biomass ratio (e.g., 1 : 2.5) is suggested to increase the concentration of PFRs (i.e., to accelerate hydrolysis and radical reaction mechanisms). On the other hand, using low water content could lead to a reduction in the pyrolyzation rate, which would require long residence times to achieve complete biomass pyrolysis. In terms of thermal pyrolysis conditions, pyrolytic temperature (relative to the heating rate and residence time) plays a critical role in determining characteristics/morphological structures of BC products by controlling H/C and O/C ratios (Fig. 4) (Lee et al., 2013). An increase in pyrolysis temperature (>500°C) can reduce catalytic activities in biorefineries as it causes a significant reduction in the BC yield, H/C and O/C ratios, porosity, and Brønsted acid sites (Cha et al., 2016). The fast pyrolysis driven at high temperatures can also reduce the yield of porous BC while increasing the yield of bio-oil (heating value of 17 MJ kg-1) and hydrochar with poor texture properties. This observation is due to accelerated pyrolysis reaction mechanisms (decomposition/depolymerization (cracking) of lignocellulosic biomass, inter-molecular rearrangements, decarboxylation/dehydration, aromatization, and condensation reactions) (Liu et al., 2015). On the other hand, the Brønsted bases, ash content, and carbon stability of BC formed at high pyrolytic temperatures can increase when using a feedstock with high pH and cellulose content (e.g., seaweeds, manures, and crop residue) (Kumar et al., 2020b; Cha et al., 2016). These features are useful in the adsorption removal of heavy metals and acid dyes (i.e., water pollution control). An overview of the parameters controlling the pyrolysis reaction mechanism and their associated impact on the yield and properties of BC products is given elsewhere (Kumar et al., 2020a, b; Xiu et al., 2017; Liu et al., 2015).
4. BIOCHAR-BASED CATALYSTS: ADVANTAGES VS. LIMITATIONS
In most heterogeneous catalysis, BC can serve as a promising medium to make various heterojunctions/composites with high catalytic activities, especially in photocatalysis for environmental remediation (e.g., air/water pollution control) (Kumar et al., 2020b; Lee et al., 2019, 2017; Wang et al., 2019). The construction of BC-supported heterojunctions/composites can be achieved by post-synthesis impregnation of noble metals and/or transition metal oxides NPs (e.g., Pt, Pb, Ru, Ni, TiO2, CuO2, MnOx, ZnO, FeVO4, Fe3O4, and BiOX) onto the BC surface through sol-gel, sonochemistry, thermal polycondensation, solvothermal, and hydrolysis methods (Mian and Liu, 2018).
Among these post-synthesis strategies, the solvent-free-ultrasonic approach is recommended as an effective clean impregnation method. However, the use of high-input ultrasound waves could cause cracking in the BC structure (i.e., reduce the quality and performance of BC-based catalysts). In terms of the techno-economic aspect, solvothermal/thermal condensation methods are ineffective options to develop BC-based catalysts due to the high energy consumption (high cost), high probability for agglomeration of catalysts, and the risk of secondary waste generation (large quantities of solvents used in synthesis and washing procedures). To overcome such disadvantages, the pre-synthesis modification of raw biomass can be used, followed by co-calcination/carbonization under nitrogen flow at low temperature (100-200°C) for a long reaction time (10-38 h) (Kumar et al., 2020b; Xiong et al., 2017). These pre-synthesis modification options are frequently used to increase the number of active sites and surface functionalities in the obtained BC catalysts through (i) doping with semiconducting or noble metals (e.g., Pt(OH)2, Pd(OH)2, Ni(NO3)2, Sn(OH)4, Cu(OH)2, Zn(NO3)2, and Co(OH)4) needed for improving thermo-/photo-catalysis and (ii) anchoring Lewis acid/base sites (viz. sulfonation, phosphorylation, amination, oxidation, acid/base treatment, ionic liquid grafting, or other hetero-atom doping) required to promote biorefinery/acid-base catalytic reactions. It should be noted that all the impregnation/modification methods mentioned above can alter the surface functionalities of BC-based catalysts due to their active role in the binding of newly loaded metal/metal oxides or other functional sites anchored on the surfaces. Although pre-/post-synthesis modification methods appear to significantly affect the physicochemical features of the BC support and its inherited catalytic sites, the performance of BC-based catalysts was generally covered without considering such critical factors in almost all systematic reviews made in this area to date.
BC can play an active role to improve the catalytic activities of heterogeneous BC-based heterojunction/composite catalysts by increasing the surface area and porous structures (pore volume and pore size distribution), providing the required accelerated mass transport and increasing the binding capacity of target organic/inorganic reactants (Fig. 5) making them closer to surface catalytic sites (Do Minh et al., 2020). As electroconductive carbon, the use of BC supports can also improve the photocatalysis via (i) shuttling the flow of excited electrons (e-) to suppress the fast recombination of e-/h+ pairs and (ii) improving the optical response of incorporated photocatalysts in the UV-visible region (Mian and Liu, 2018). The high density of O, N, and S-type functional moieties on BC surfaces also enhances acid-driven catalytic reactions in biorefinery reactions (e.g., biofuel production: trans-esterification, hydrolysis, and dehydration reaction) (Kumar et al., 2020b; Wang et al., 2019). Similarly, the synthesis of BC with functionalities rich in p-block heteroatoms (e.g., N, P, S, and B) is also preferred in the design of visible/solar-driven BC-based photocatalysts. This is because these non-metal elements can modulate the electronic states (conduction band) of BC to promote electro-photo-chemical catalysis (Do Minh et al., 2020). Hence, besides inherited functionalities, doping surface functionalities onto pristine BC materials by pre-/post-synthesis modification strategies is an effective route to increase the catalytic activities of BC-based catalysts in biorefinery (Xiong et al., 2017) and photocatalysis applications (Cui et al., 2020; Mian and Liu, 2018). Further, the interaction between the impregnated catalytic sites and graphitic carbon with a high dispersion of minerals significantly enhanced the catalytic activity of BC-based catalysts. However, in some cases, the high mineral content (e.g., Si) in raw biomass could have a negative role in the catalytic activities of BC catalysts as it promotes alkali/transition metal sintering at high temperatures (agglomeration) when using steam-containing thermal pyrolysis (Cheng and Li, 2018). On the other hand, the synthesis of BC through the gasification of biomass in CO2 can address such a drawback and increase metal dispersion.
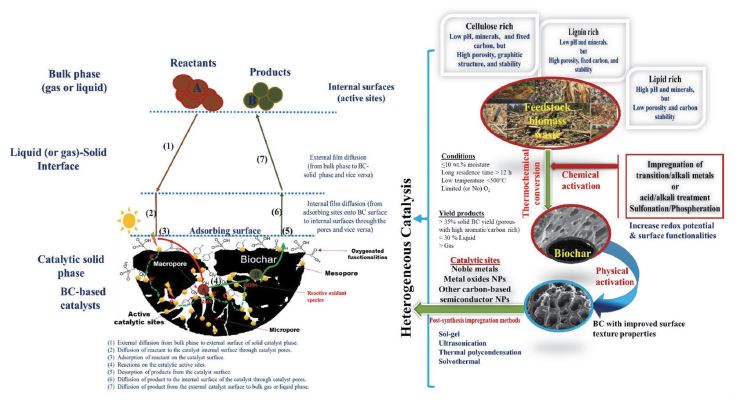
Schematic for the synthesis/activation of BC-based catalysts and the associated catalytic reaction steps in heterogeneous catalysis.
The texture properties of BC are a critical part of enhancing the catalytic activities by accelerating the mass diffusion step, as seen in Fig. 5. It was noted that most synthesized bare BC supports have lower surface areas (1.5-241 m2 g-1) and pore volumes (0.01-0.39 m3 g-1) than other carbon supports (like activated carbon, graphene oxides, and carbon nanotubes). Further, all post-impregnation/surface functionalization methods are known to reduce the surface porosity, and surface area of BC supports to a certain degree due to pore blocking with anchored materials with non-metals, noble metals, or metal/metal oxides on the surface. To resolve this problem, physical activation of BC by steam treatment under high-temperature conditions (≥700°C for 1-8 h) is frequently proposed as a favorable post-modification option to increase the surface area and to increase the channel (pore) volume (e.g., a factor of >122 improvements) (Xiong et al., 2017). More importantly, the co-activation method (viz. pre-chemical and post-physical activation) showed a more preferable synthesis scenario to improve the physicochemical/texture features of the final BC supports (in terms of mesoporosity, high surface area >1000 m2 g-1, and an increase in the number of Lewis acid/base and metal catalytic sites) (Liu et al., 2015). Nonetheless, we should keep in mind that such a physical activation process is energy-intensive and time-consuming, leading to an increase in the production cost of BC-supported catalysts.
Among catalysts reported in the energy field (Fig. 6, e.g., biomass hydrolysis reactions, esterification, and methanation), the improved dispersibility (>70%) of Ru catalyst on activated BC (ABC) support with a high surface area of 728-757 m2 g-1 was highly effective in the methanation reaction (e.g., conversion of 55% CO2: 97% CO to CH4, onto Ru/ABC with 92% selectivity) (Xiong et al., 2017). Sulfonated biochar (BC-SO3H) has been recommended strongly for promoting the esterification/transesterification reactions of free fatty acid (FFA) in acid oils to biodiesel, with a conversion efficiency in the range of 97-98% (using refined microalgal oil at 100°C) to 77-89% (using waste vegetable oils at 60°C) (Xiong et al., 2017). These results indicated that the effectiveness of BC catalysts in biodiesel production is mainly dependent on the oil source and operation conditions. Also, it should be noted that the high catalytic performance of BC-SO3H in esterification/transesterification reactions was attributed to the presence of a high acid site density (like -SO3H, -COOH, and -OH groups) on the BC surface (referred to as a solid acid catalyst). Likewise, it was found that the BC-SO3H catalyst had high performance in the catalytic hydrolysis of biomass to value-added glucose product, with yield efficiencies at 19.8% (Liu et al., 2015) and 85.4% (Shan et al., 2020). This difference in the catalytic activity of BC-SO3H is attributed to two main reasons: (i) the difference in the loaded density of -SO3H sites (e.g., 1.99 and 0.196 mmol g-1) in the BC, along with its textural characteristics and (ii) the change in catalytic conditions (e.g., mass: catalyst ratio, reaction temperature, and time). To improve the properties of BC-SO3H acid catalysts, the use of gaseous sulfonation (using >20% SO3 gas) is a preferable option to that of liquid (95% H2SO4) sulfonation to increase the loaded density of -SO3H sites on BC, while the latter is favored to improve the textural features of BC over the former (Lee et al., 2017).
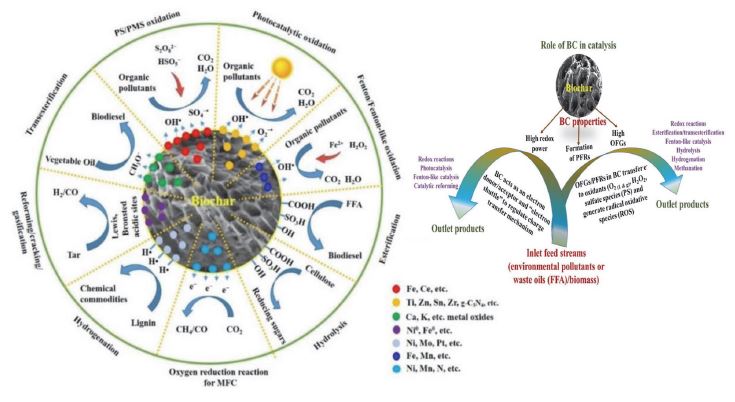
The catalytic mechanisms of BC-based catalysts in environmental and energy applications and the role of BC surfaces in these heterogeneous catalytic reactions. Adapted from Ref. (Shan et al., 2020).
For catalytic applications in environmental engineering (Fig. 6), BC-based catalysts are useful to induce various advanced oxidation processes (AOPs, like Fenton-like reaction, sonocatalysis, electrocatalysis, photocatalysis, and redox reactions) of multiple emergent organic pollutants (Do Minh et al., 2020; Lyu et al., 2020; Wang et al., 2019). Among reported BC-based catalysts, one decorated with graphitic carbon nitride (BC@g-C3N4) as core-shell spheres was found to significantly improve visible-driven photocatalysis against bisphenol A (91% removal with a rate of 8.86×10-3 min-1) and provide good stability up to 5 cycles (Lyu et al., 2020). In this case, the BC acted as a charge carrier to stabilize the active species and enhance e-/h+ pair separation in g-C3N4 (i.e., prolonged exciton lifespan) to accelerate photo-redox reactions. In sonocatalysis, the synthesis of lanthanide CeO2-H@BC catalysts exhibited a high catalytic capacity for enhanced degradation (98.5%) of organic dye (e.g., RR84) under mild sonolysis conditions (at 450 W and pH 6.5) for 1 h. Further, the increased FBR concentration in BC with high iron (Fe) content could improve oxidative redox reactions (via Fenton-like catalysis) against various organic contaminants in water solutions in the presence of homogenous oxidants (like H2O2, HSO5-, or S2O82-) (Shan et al., 2020). More interestingly, BC materials have recently been utilized either as supports or catalysts in many coupling catalytic processes for the practical treatment of real industrial wastewater, such as in bio-electrochemical, bio-electro-Fenton, and photo-bio-electrochemical combined technologies (Do Minh et al., 2020). Detailed catalytic performance analysis of BC-based catalysts was not the key aim of this work. Hence, interested readers may refer to the reviews organized to evaluate the catalytic performances of many synthesized BC-based catalysts in energy (biofuel/biorefinery) production (Kumar et al., 2020b; Xiong et al., 2017; Qian et al., 2015) and environmental protection (Do Minh et al., 2020; Lyu et al., 2020).
From the above findings, we concluded that designing a broadly active BC catalyst for different catalytic reactions is a challenging process, either in energy or environmental fields. The major hurdles in the design of the BC catalyst are the complex nature of biomass waste, the activation process, and establishing pyrolytic operation conditions. However, the low cost and sustainability of raw biomass substrates are the keys to increasing the probability of engineering BC catalysts as industrially viable options to replace traditional catalysts (like metal-based or other carbon-based catalysts) in environmental and energy fields. Therefore, one needs to consider the following aspects in the development of commercial BC-based heterogeneous catalysts: (i) selection of appropriate raw biomass (determine elemental composition) with low initial cost and (ii) optimization of synthesis conditions for BC (controlled surface and texture features) prior to the pre-/post-impregnation/functionalization methods.
5. COMMERCIALIZATION: TECHNO-ECONOMIC ASPECT AND FUTURE PERSPECTIVE
Before realizing the scalable production of commercialized BC catalysts, it is essential to provide a holistic techno-economic analysis related to the thermochemical conversion of biomass to BC-driven catalytic materials and their environmental impacts. To accurately compute the techno-economic aspects, one should consider four parameters during BC catalyst synthesis: (i) the collection, chipping, and pelletization of raw feedstock precursors, (ii) processing, activation, or modification processes (i.e., pre-/post-synthesis strategies), (iii) energy consumption during carbonization set up (conversion method), and (iv) economic (based on mass/energy balances) and environmental (greenhouse gas emission (GHG) as an indicator) performance indicators (inputs-to-outputs) observed during synthesis (Casson Moreno et al., 2020). In terms of economic performance, the estimated average investment costs were in the ranges of 11.35-24.80 USD/ton for biomass collection and 380-580 USD/ton for production of BC using pyrolysis/gasification processes (Do Minh et al., 2020). The low cost-production of BC is due to the exothermic nature of the pyrolysis process (i.e., requiring a low energy of ≈85 MJ ton-1 biomass) (Kumar et al., 2020a). The mass-to-energy balance was also approximated at 30.3 MJ kg-1 when using olive trees with a high heating value under thermal conversion at 700°C with a mass flow of 6 kg h-1 (Casson Moreno et al., 2020). Globally, the revenue-cost ratio of bare BC (sale/production) is positive at 1.02, indicating the significant benefits of BC synthesis from an economic viewpoint. BC catalysts made via thermal pyrolysis are also expected to have a positive impact on the environment if the pyrolytic char by-products (or spent BC catalysts) are not disposed of in a landfill but are used as a soil amendment for non-edible agriculture.
The fate-life cycle of BC materials in their applications could also show substantial benefits for both environmental and economic performance indicators through reutilization as a reactive carbon for capturing GHG in the environment and as fertilizers in soil (Matuštík et al., 2020). In China (Guizhou and Henan provinces), a new pilot-scale rotary kiln has recently been built for the coproduction of heat and BC (600 kg h-1) using the syngas generated during the biomass pyrolytic process. Other Chinese companies operated a semi-closed sub-high-temperature anoxic carbonization furnace for the coproduction of BC, bio-oil, heat, and electricity during thermopyrolysis of biomass (Chen et al., 2019). Using these technologies, the produced energy was higher than that of energy required for the thermal pyrolysis of biomass- to-BC catalysts (i.e., positive net energy). Accordingly, the profits of biomass-to-BC catalyst production (heat, bio-oil, and electricity generation) could compensate for the GHG generated during the pyrolysis process. Results also revealed that the scenario of co-production of BC and biofuel (bio-oil and syngas) during pyrolysis provided a revenue of 24.2 million USD based on historic energy market prices. The total revenue was 5.5 million USD in terms of the biomass waste management scenario (Kumar et al., 2020a). In environmental protection applications, the projected costs for employing BC catalysts in wastewater treatment (1000 gallons) were approximately 14, 8648, and 15536 USD for Fenton-like reactions, photocatalysis, and sonocatalytic processes, respectively (Wang et al., 2019). The high estimated treatment cost in photocatalysis and sonocatalytic processes may mainly reflect high electrical consumption and the need to purchase experimental tools (like ultrasound and UV-vis lamps). In the Fenton reaction, the homogenous oxidant represents the major part of the total treatment cost. These findings suggest the beneficial aspects of BC catalysts, as cost-effective and sustainable materials in environmental protection and energy applications, especially at a large industrial scale.
As a net conclusion, the techno-economic analysis of BC production suggests it has a bright future for large-scale applications of BC catalysts as inexpensive heterogeneous catalysts. However, the performance of those catalysts in environmental/energy fields is still in the research and development stage. This is mainly due to the low catalytic selectivity and stability of BC catalysts with complex surface chemistry (e.g., chemical/elemental composition, texture features, surface functionalities, redox potential, and total acidity), which is dependent on the factors controlling the synthesis (See Section 3). Hence, it is fundamentally important to optimize those parameters for scalable production and commercialization to maximize the techno-economic efficiency and surface chemistry of BC catalysts for enhanced catalytic performance. To meet such a goal, one should consider and understand the synergistic effect between the elemental composition of biomass and pyrolysis conditions (e.g., for synergistic increases in the number of generated catalytic sites and surface functionality (OFGs and PFRs) in pristine BC materials). Further, a deep understanding of the role of the co-activation (physical and chemical activation) in improving texture properties of pristine BC is crucial, as such information should help enable the design of inexpensive and effective BC catalysts for target catalytic reactions without the need for post-synthesis modification that can increase production cost while limiting scaled production.
6. CONCLUSIONS
Biochar (BC) and its composites have shown promising potential for applications as solid catalysts in various heterogeneous catalytic reactions (e.g., acid-base reaction, biorefinery reactions, hydrogenation, methanation, photo-electro-catalysis, bio-electrochemical reactions, and advanced oxidation reactions). Nonetheless, BC-based catalysts are still confined to the research and development stage (e.g., lab-scale studies) in which their performance is assessed under favorably adjusted experimental conditions rather than what is actually encountered under real-world conditions. Further, it was found that the basic properties of the same BC-based catalysts (e.g., inherited functionalities/catalytic sites) were significantly different from one report to another due to the subtle differences in the synthesis environments of pristine BC. As such, it is difficult to assess the best-performing BC-based catalysts for industrial applications.
The direct use of pristine BC as a catalyst is not possible for all types of catalysis but can be realized in some heterogeneous catalysis, especially in energy generation applications (e.g., catalytic pyrolysis/gasification, esterification/transesterification, and hydrolysis reactions). However, the direct use of BC in those catalytic reactions depends on the conditions of thermochemical conversion (e.g., from waste biomass to BC) and their effect on the generated active sites (e.g., high surface acidity, OFGs, and PFRs sites). In this context, it should be noted that the presence of active sites in BC-based catalysts is dependent on the following factors: (i) the relationship between the nature of the precursor (e.g., the elemental composition of waste biomass) and pyrolysis conditions, (ii) the actual effect of such interrelated factors on the physicochemical properties of BC (i.e., texture and elemental active sites), and (iii) the effect of pre-synthesis conditions (e.g., pre-chemical activation and moisture/water content of biomass) on the surface chemistry of BC catalysts (total acidity, OFGs, and PFRs sites).
In environmental and air pollution control, BC is frequently utilized as a low-cost template to design various heterojunctions/composites to enhance reactivities in diverse processes (e.g., thermocatalysis, photocatalysis, electrocatalysis, and redox reactions) for pollution control. In this respect, many different types of post-synthesis modification (PSM) methods have been utilized to impregnate/incorporate organic catalytic nanoparticles, transition metals/metal oxides, and non-metal active sites on the surface of the BC support. The catalytic performance of those BC-based composites employed in environmental catalysis was dictated by the combined effects of several factors such as: (i) the physicochemical features of BC (surface functionality, conductivity, and texture properties) and (ii) the catalytic activity of catalysts doped/incorporated on the BC surfaces. In contrast, most PSM strategies led to changes in the pristine BC surface properties with an increase in the production cost of BC-based catalysts that can be crucial in the catalytic treatment of environmental pollutants. For example, as a semiconductor with a graphene-like skeleton, BC can serve as (i) an electron reservoir to improve charge separation of electrons and holes to carry out visible- light-driven photocatalysis and (ii) an adsorbent to capture organic/inorganic contaminants for the acceleration of catalytic degradation/reduction during photo/electro-catalysis (or redox reactions). Hence, prior to the design of BC-based catalysts for environmental application, the effect of the PSM strategy should be considered to ensure that the physicochemical features of BC supports can be improved most cost-effectively.
From a techno-economic viewpoint, large-scale thermochemical conversion of biomass to BC product is favored because it provides many environmental/economic advantages in terms of waste management, production of eco-friendly low-cost value-added products, abatement of environmental pollution and GHGs, high energy generation, and soil reclamation. Accordingly, future research should consider the following technical and economic aspects to improve the production of BC-based catalysts for industrial-scale applications in environmental/energy fields: (1) optimization of the synthesis conditions for the BC-based catalysts should be performed to maximize their catalytic performance with the lowest production cost, (2) future research should be carried out under industrially relevant experimental conditions (e.g., similar to target industrial operation) to maximize the applicability of BC-based catalysts in industry, (3) the PSM strategies should be employed with the least negative impact on the inherent properties of BC templates and to help develop BC-based composite catalysts, and (4) the development of BC-based catalysts and their composites for future catalytic studies should include environmental and techno-economic assessments that are compared to other nanoscale or commercial catalysts produced at industrial scales. In summary, a systematic understanding of the parameters affecting the inherent properties of biochar materials (e.g., synthesis procedures, biomass precursors, and modification strategies) will assist in designing efficient biochar-based catalysts for environmental applications, in particular for air pollution control.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ITC (MSIT) of Korean government (Grant No: 2021R1A3B1068304).
References
-
Abe, M., Kawashima, K., Kozawa, K., Sakai, H., Kaneko, K. (2000) Amination of activated carbon and adsorption characteristics of its aminated surface. Langmuir, 16, 5059-5063.
[https://doi.org/10.1021/la990976t]

-
Abhishek, K., Srivastava, A., Vimal, V., Gupta, A.K., Bhujbal, S.K., Biswas, J.K., Singh, L., Ghosh, P., Pandey, A., Sharma, P. (2022) Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Science of The Total Environment, 853, 158562.
[https://doi.org/10.1016/j.scitotenv.2022.158562]

-
Amusat, S.O., Kebede, T.G., Dube, S., Nindi, M.M. (2021) Ball-milling synthesis of biochar and biochar-based nanocomposites and prospects for removal of emerging contaminants: A review. Journal of Water Process Engineering, 41, 101993.
[https://doi.org/10.1016/j.jwpe.2021.101993]

-
Anto, S., Sudhakar, M.P., Ahamed, T.S., Samuel, M.S., Mathimani, T., Brindhadevi, K., Pugazhendhi, A. (2021) Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 285, 119205.
[https://doi.org/10.1016/j.fuel.2020.119205]

-
Awasthi, M.K. (2022) Engineered biochar: A multifunctional material for energy and environment. Environmental Pollution, 298, 118831.
[https://doi.org/10.1016/j.envpol.2022.118831]

-
Balajii, M., Niju, S. (2019) Biochar-derived heterogeneous catalysts for biodiesel production. Environmental Chemistry Letters, 17, 1447-1469.
[https://doi.org/10.1007/s10311-019-00885-x]

-
Bhavani, P., Hussain, M., Park, Y.K. (2022) Recent advancements on the sustainable biochar based semiconducting materials for photocatalytic applications: A state of the art review. Journal of Cleaner Production, 330, 129899.
[https://doi.org/10.1016/j.jclepro.2021.129899]

-
Bolan, N., Hoang, S.A., Beiyuan, J., Gupta, S., Hou, D., Karakoti, A., Joseph, S., Jung, S., Kim, K.H., Kirkham, M.B., Kua, H.W., Kumar, M., Kwon, E.E., Ok, Y.S., Perera, V., Rinklebe, J., Shaheen, S.M., Sarkar, B., Sarmah, A.K., Singh, B.P., Singh, G., Tsang, D.C.W., Vikrant, K., Vithanage, M., Vinu, A., Wang, H., Wijesekara, H., Yan, Y., Younis, S.A., Van Zwieten, L. (2022) Multifunctional applications of biochar beyond carbon storage. International Materials Reviews, 67, 150-200.
[https://doi.org/10.1080/09506608.2021.1922047]

-
Casson Moreno, V., Iervolino, G., Tugnoli, A., Cozzani, V. (2020) Techno-economic and environmental sustainability of biomass waste conversion based on thermocatalytic reforming. Waste Management, 101, 106-115.
[https://doi.org/10.1016/j.wasman.2019.10.002]

-
Cha, J.S., Kim, Y.-M., Choi, Y.J., Rhee, G.H., Song, H., Jeon, B.-H., Lam, S.S., Khan, M.A., Lin, K.-Y.A., Chen, W.-H. (2022) Mitigation of hazardous toluene via ozone-catalyzed oxidation using MnOx/Sawdust biochar catalyst. Environmental Pollution, 312, 119920.
[https://doi.org/10.1016/j.envpol.2022.119920]

-
Cha, J.S., Park, S.H., Jung, S.C., Ryu, C., Jeon, J.K., Shin, M.C., Park, Y.K. (2016) Production and utilization of biochar: A review. Journal of Industrial and Engineering Chemistry, 40, 1-15.
[https://doi.org/10.1016/j.jiec.2016.06.002]

-
Chatterjee, R., Sajjadi, B., Chen, W.-Y., Mattern, D.L., Egiebor, N.O., Hammer, N., Raman, V. (2019) Low frequency ultrasound enhanced dual amination of biochar: a nitrogen-enriched sorbent for CO2 capture. Energy & Fuels 33, 2366-2380.
[https://doi.org/10.1021/acs.energyfuels.8b03583]

-
Chatterjee, R., Sajjadi, B., Chen, W.-Y., Mattern, D.L., Hammer, N., Raman, V., Dorris, A. (2020) Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Frontiers in Energy Research, 8, 85.
[https://doi.org/10.3389/fenrg.2020.00085]

-
Chen, W., Meng, J., Han, X., Lan, Y., Zhang, W. (2019) Past, present, and future of biochar. Biochar, 1, 75-87.
[https://doi.org/10.1007/s42773-019-00008-3]

-
Cheng, F., Li, X. (2018) Preparation and application of biochar-based catalysts for biofuel production. Catalysts, 8, 1-35.
[https://doi.org/10.3390/catal8090346]

-
Cui, J., Zhang, F., Li, H., Cui, J., Ren, Y., Yu, X. (2020) Recent Progress in Biochar-Based Photocatalysts for Wastewater Treatment: Synthesis, Mechanisms, and Applications. Applied Sciences, 10, 1019.
[https://doi.org/10.3390/app10031019]

-
Ding, S., Liu, Y. (2020) Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 260, 116382.
[https://doi.org/10.1016/j.fuel.2019.116382]

-
Do Minh, T., Song, J., Deb, A., Cha, L., Srivastava, V., Sillanpää, M. (2020) Biochar based catalysts for the abatement of emerging pollutants: A review. Chemical Engineering Journal, 394, 124856.
[https://doi.org/10.1016/j.cej.2020.124856]

-
Fawzy, S., Osman, A.I., Yang, H., Doran, J., Rooney, D.W. (2021) Industrial biochar systems for atmospheric carbon removal: a review, Environmental Chemistry Letters. Springer International Publishing.
[https://doi.org/10.1007/s10311-021-01210-1]

-
Ghosh, S.K. (2016) Biomass & Bio-waste Supply Chain Sustainability for Bio-energy and Bio-fuel Production. Procedia Environmental Sciences, 31, 31-39.
[https://doi.org/10.1016/j.proenv.2016.02.005]

- GLOBAL BIOENERGY STATISTICS-World Bioenergy Association (2019)
-
Guo, F., Jia, X., Liang, S., Zhou, N., Chen, P., Ruan, R. (2020) Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification. Bioresource Technology, 298, 122263.
[https://doi.org/10.1016/j.biortech.2019.122263]

-
Guo, S., Li, Y., Wang, Y., Wang, L., Sun, Y., Liu, L. (2022) Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Science & Technology, 4, 100059.
[https://doi.org/10.1016/j.ccst.2022.100059]

-
Gwenzi, W., Chaukura, N., Wenga, T., Mtisi, M. (2021) Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Science of the Total Environment
[https://doi.org/10.1016/j.scitotenv.2020.142249]

-
Hu, Z., Liu, W. (2020) Conversion of biomasses and copper into catalysts for photocatalytic CO2 reduction. ACS Applied Materials & Interfaces, 12, 51366-51373.
[https://doi.org/10.1021/acsami.0c13323]

-
Huang, J., Zhang, H., Tan, Q., Li, L., Xu, R., Xu, Z., Li, X. (2021) Enhanced conversion of CO2 into O2-free fuel gas via the Boudouard reaction with biochar in an atmospheric plasmatron. Journal of CO2 Utilization, 45.
[https://doi.org/10.1016/j.jcou.2020.101429]

-
Kumar, A., Saini, K., Bhaskar, T. (2020a) Hydochar and biochar: Production, physicochemical properties and techno-economic analysis. Bioresource Technology, 310, 123442.
[https://doi.org/10.1016/j.biortech.2020.123442]

-
Kumar, A., Saini, K., Bhaskar, T. (2020b) Advances in design strategies for preparation of biochar based catalytic system for production of high value chemicals. Bioresource Technology, 299, 122564.
[https://doi.org/10.1016/j.biortech.2019.122564]

-
Kumar, A., Kumar, A., Sharma, G., Al-Muhtaseb, A.H., Naushad, M., Ghfar, A.A., Guo, C., Stadler, F.J. (2018) Biochartemplated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chemical Engineering Journal, 339, 393-410.
[https://doi.org/10.1016/j.cej.2018.01.105]

-
Kumar, M., Xiong, X., Sun, Y., Yu, I.K.M., Tsang, D.C.W., Hou, D., Gupta, J., Bhaskar, T., Pandey, A. (2020c) Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Advanced Sustainable Systems, 4, 1900149.
[https://doi.org/10.1002/adsu.201900149]

-
Lee, H.W., Kim, J.K., Park, Y.K. (2018) Adsorptive removal of odour substances and NO and catalytic esterification using empty fruit bunch derived biochar. Carbon Letters, 28, 81-86.
[https://doi.org/10.5714/CL.2018.28.081]

-
Lee, H.W., Lee, H., Kim, Y.M., Park, R., Park, Y.K. (2019) Recent application of biochar on the catalytic biorefinery and environmental processes. Chinese Chemical Letters, 30, 2147-2150.
[https://doi.org/10.1016/j.cclet.2019.05.002]

-
Lee, J., Kim, K.H., Kwon, E.E. (2017) Biochar as a Catalyst. Renewable and Sustainable Energy Reviews, 77, 70-79.
[https://doi.org/10.1016/j.rser.2017.04.002]

-
Lee, Y., Eum, P.R.B., Ryu, C., Park, Y.K., Jung, J.H., Hyun, S. (2013) Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresource Technology, 130, 345-350.
[https://doi.org/10.1016/j.biortech.2012.12.012]

-
Li, L., Song, J., Yao, X., Huang, G., Liu, Z., Tang, L. (2012) Adsorption of volatile organic compounds on three activated carbon samples: Effect of pore structure. Journal of Central South University, 19, 3530-3539.
[https://doi.org/10.1007/s11771-012-1439-x]

-
Li, X., Qian, X., An, X., Huang, J. (2019) Preparation of a novel composite comprising biochar skeleton and “chrysanthemum” g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde. Applied Surface Science, 487, 1262-1270.
[https://doi.org/10.1016/j.apsusc.2019.05.195]

-
Li, Y., Liu, Z., Li, Z., Wang, Q. (2022) Renewable biomass-derived carbon-supported g-C3N4 doped with Ag for enhanced photocatalytic reduction of CO2. Journal of Colloid and Interface Science, 606, 1311-1321.
[https://doi.org/10.1016/j.jcis.2021.08.176]

-
Lin, F., Gao, M., Wang, Y., Yu, J., Gao, C., Zang, S. (2022) Enhanced mass transfer with amphiphilic CoOx/biochar for photocatalytic CO2 conversion. Applied Surface Science, 605, 154612.
[https://doi.org/10.1016/j.apsusc.2022.154612]

-
Liu, W.J., Jiang, H., Yu, H.Q. (2019) Emerging applications of biochar-based materials for energy storage and conversion. Energy and Environmental Science, 12, 1751-1779.
[https://doi.org/10.1039/C9EE00206E]

-
Liu, W.J., Jiang, H., Yu, H.Q. (2015) Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chemical Reviews, 115, 12251-12285.
[https://doi.org/10.1021/acs.chemrev.5b00195]

-
Lourenço, M.A.O., Zeng, J., Jagdale, P., Castellino, M., Sacco, A., Farkhondehfal, M.A., Pirri, C.F. (2021) Biochar/zinc oxide composites as effective catalysts for electrochemical CO2 reduction. ACS Sustainable Chemistry & Engineering, 9, 5445-5453.
[https://doi.org/10.1021/acssuschemeng.1c00837]

-
Lyu, H., Zhang, Q., Shen, B. (2020) Application of biochar and its composites in catalysis. Chemosphere 240, 124842.
[https://doi.org/10.1016/j.chemosphere.2019.124842]

-
Matuštík, J., Hnátková, T., Kočí, V. (2020) Life cycle assessment of biochar-to-soil systems: A review. Journal of Cleaner Production, 259, 120998.
[https://doi.org/10.1016/j.jclepro.2020.120998]

-
McBeath, A.V., Wurster, C.M., Bird, M.I. (2015) Influence of feedstock properties and pyrolysis conditions on biochar carbon stability as determined by hydrogen pyrolysis. Biomass and Bioenergy 73, 155-173.
[https://doi.org/10.1016/j.biombioe.2014.12.022]

- McWilliams, A. (2015) Catalysts for Environmental and Energy Applications, CHM020E. BCC Research.
-
Meng, F., Song, M., Wei, Y., Wang, Y. (2019) The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environmental Science and Pollution Research, 26, 7195-7204.
[https://doi.org/10.1007/s11356-019-04190-6]

-
Mian, M.M., Liu, G. (2018) Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Advances, 8, 14237-14248.
[https://doi.org/10.1039/C8RA02258E]

-
Monga, D., Shetti, N.P., Basu, S., Raghava Reddy, K., Badawi, M., Bonilla-Petriciolet, A., Aminabhavi, T.M. (2022) Engineered biochar: A way forward to environmental remediation. Fuel 311.
[https://doi.org/10.1016/j.fuel.2021.122510]

-
Qian, K., Kumar, A., Zhang, H., Bellmer, D., Huhnke, R. (2015) Recent advances in utilization of biochar. Renewable and Sustainable Energy Reviews, 42, 1055-1064.
[https://doi.org/10.1016/j.rser.2014.10.074]

-
Rangarajan, G., Jayaseelan, A., Farnood, R. (2022) Photocatalytic reactive oxygen species generation and their mechanisms of action in pollutant removal with biochar supported photocatalysts: A review. Journal of Cleaner Production, 131155.
[https://doi.org/10.1016/j.jclepro.2022.131155]

-
Rezaee, A., Rangkooy, H., Khavanin, A., Jafari, A.J. (2014) High photocatalytic decomposition of the air pollutant formaldehyde using nano-ZnO on bone char. Environmental Chemistry Letters, 12, 353-357.
[https://doi.org/10.1007/s10311-014-0453-7]

-
Shaheen, S.M., Antoniadis, V., Shahid, M., Yang, Y., Abdelrahman, H., Zhang, T., Hassan, N.E.E., Bibi, I., Niazi, N.K., Younis, S.A. (2022) Sustainable applications of rice feedstock in agro-environmental and construction sectors: a global perspective. Renewable and Sustainable Energy Reviews, 153, 111791.
[https://doi.org/10.1016/j.rser.2021.111791]

-
Shan, R., Han, J., Gu, J., Yuan, H., Luo, B., Chen, Y. (2020) A review of recent developments in catalytic applications of biochar-based materials. Resources, Conservation and Recycling, 162, 105036.
[https://doi.org/10.1016/j.resconrec.2020.105036]

-
Shrestha, P., Chun, D.D., Kang, K., Simson, A.E., Klinghoffer, N.B. (2022) Role of Metals in Biochar Production and Utilization in Catalytic Applications: A Review. Waste and Biomass Valorization 13, 797-822.
[https://doi.org/10.1007/s12649-021-01519-6]

- Size, C.M. (2020) Share & Trends Analysis Report by Raw Material (Chemical Compounds, Zeolites, Metals), By Product (Heterogeneous, Homogeneous), By Application, By Region, And Segment Forecasts, 2020-2027, Catalysts & Enzymes, Catalyst Market Size & Share, Industry Report [WWW Document].
-
Smith, P. (2016) Soil carbon sequestration and biochar as negative emission technologies. Global Change Biology, 22, 1315-1324.
[https://doi.org/10.1111/gcb.13178]

-
Thangarajan, R., Bolan, N.S., Kunhikrishnan, A., Wijesekara, H., Xu, Y., Tsang, D.C.W., Song, H., Ok, Y.S., Hou, D. (2018) The potential value of biochar in the mitigation of gaseous emission of nitrogen. Science of the Total Environment, 612, 257-268.
[https://doi.org/10.1016/j.scitotenv.2017.08.242]

-
Thomazini, A., Spokas, K., Hall, K., Ippolito, J., Lentz, R., Novak, J. (2015) GHG impacts of biochar: Predictability for the same biochar. Agriculture, Ecosystems & Environment, 207, 183-191.
[https://doi.org/10.1016/j.agee.2015.04.012]

-
Tripathi, N., Hills, C.D., Singh, R.S., Atkinson, C.J. (2019) Biomass waste utilisation in low-carbon products: harnessing a major potential resource. Npj Climate and Atmospheric Science, 2.
[https://doi.org/10.1038/s41612-019-0093-5]

-
Uchimiya, M., Wartelle, L.H., Lima, I.M., Klasson, K.T. (2010) Sorption of deisopropylatrazine on broiler litter biochars. Journal of Agricultural and Food Chemistry, 58, 12350-12356.
[https://doi.org/10.1021/jf102152q]

-
Wang, R.Z., Huang, D.L., Liu, Y.G., Zhang, C., Lai, C., Wang, X., Zeng, G.M., Gong, X.M., Duan, A., Zhang, Q., Xu, P. (2019) Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chemical Engineering Journal, 371, 380-403.
[https://doi.org/10.1016/j.cej.2019.04.071]

-
Xiong, X., Yu, I.K.M., Cao, L., Tsang, D.C.W., Zhang, S., Ok, Y.S. (2017) A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresource Technology, 246, 254-270.
[https://doi.org/10.1016/j.biortech.2017.06.163]

-
Xiu, S., Shahbazi, A., Li, R. (2017) Characterization, Modification and Application of Biochar for Energy Storage and Catalysis: A Review. Trends in Renewable Energy 3, 86-101.
[https://doi.org/10.17737/tre.2017.3.1.0033]

-
Xu, X., Kan, Y., Zhao, L., Cao, X. (2016) Chemical transformation of CO2 during its capture by waste biomass derived biochars. Environmental Pollution, 213, 533-540.
[https://doi.org/10.1016/j.envpol.2016.03.013]

-
Xu, X., Zheng, Y., Gao, B., Cao, X. (2019) N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chemical Engineering Journal, 368, 564-572.
[https://doi.org/10.1016/j.cej.2019.02.165]

-
Younis, S.A., El-Salamony, R.A., Tsang, Y.F., Kim, K.-H. (2020) Use of rice straw-based biochar for batch sorption of barium/strontium from saline water: Protection against scale formation in petroleum/desalination industries. Journal of Cleaner Production, 250, 119442.
[https://doi.org/10.1016/j.jclepro.2019.119442]

-
Younis, S.A., Kim, K.-H., Shaheen, S.M., Antoniadis, V., Tsang, Y.F., Rinklebe, J., Deep, A., Brown, R.J.C. (2021) Advancements of nanotechnologies in crop promotion and soil fertility: Benefits, life cycle assessment, and legislation policies. Renewable and Sustainable Energy Reviews, 152, 111686.
[https://doi.org/10.1016/j.rser.2021.111686]

-
Yue, X., Ma, N.L., Sonne, C., Guan, R., Lam, S.S., Van Le, Q., Chen, X., Yang, Y., Gu, H., Rinklebe, J., Peng, W. (2021) Mitigation of indoor air pollution: A review of recent advances in adsorption materials and catalytic oxidation. Journal of Hazardous Materials, 405.
[https://doi.org/10.1016/j.jhazmat.2020.124138]

-
Zhang, C., Sun, S., Xu, S., Wu, C. (2022a) CO2 capture over steam and KOH activated biochar: Effect of relative humidity. Biomass and Bioenergy 166, 106608.
[https://doi.org/10.1016/j.biombioe.2022.106608]

-
Zhang, H., Tan, Q., Huang, Q., Wang, K., Tu, X., Zhao, X., Wu, C., Yan, J., Li, X. (2022b) Boosting the Conversion of CO2 with Biochar to Clean CO in an Atmospheric Plasmatron: A Synergy of Plasma Chemistry and Thermochemistry. ACS Sustainable Chemistry & Engineering, 10, 7712-7725.
[https://doi.org/10.1021/acssuschemeng.2c01778]

-
Zhang, X., Gao, B., Creamer, A.E., Cao, C., Li, Y. (2017) Adsorption of VOCs onto engineered carbon materials: A review. Journal of Hazardous Materials, 338, 102-123.
[https://doi.org/10.1016/j.jhazmat.2017.05.013]

-
Zhang, Y., Wang, S., Feng, D., Gao, J., Dong, L., Zhao, Y., Sun, S., Huang, Y., Qin, Y. (2022c) Functional Biochar Synergistic Solid/Liquid-Phase CO2 Capture: A Review. Energy & Fuels 36, 2945-2970.
[https://doi.org/10.1021/acs.energyfuels.1c04372]

-
Zhao, Z., Wang, B., Theng, B.K.G., Lee, X., Zhang, X., Chen, M., Xu, P. (2022) Removal performance, mechanisms, and influencing factors of biochar for air pollutants: a critical review. Biochar, 4, 1-24.
[https://doi.org/10.1007/s42773-022-00156-z]